

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070409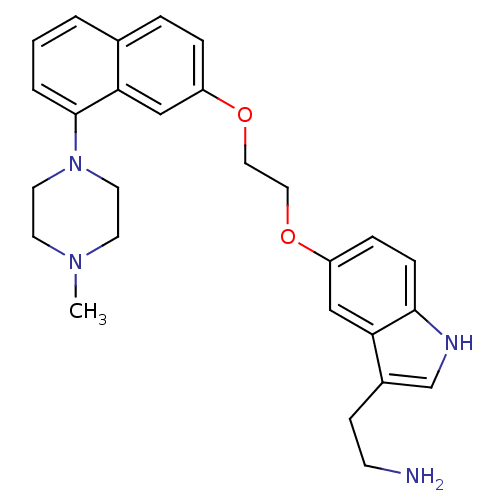 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070406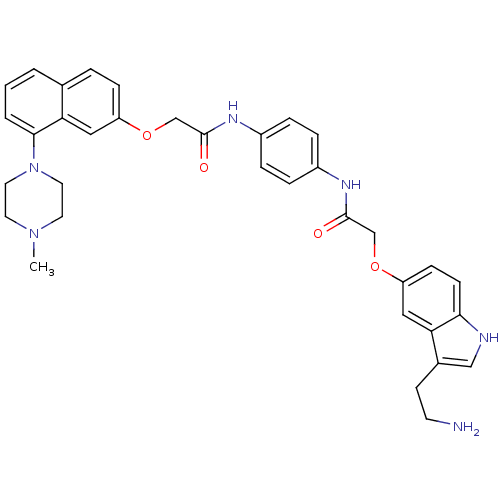 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070406 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[8-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070409 (2-(5-(2-(8-(4-methylpiperazin-1-yl)naphthalen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070408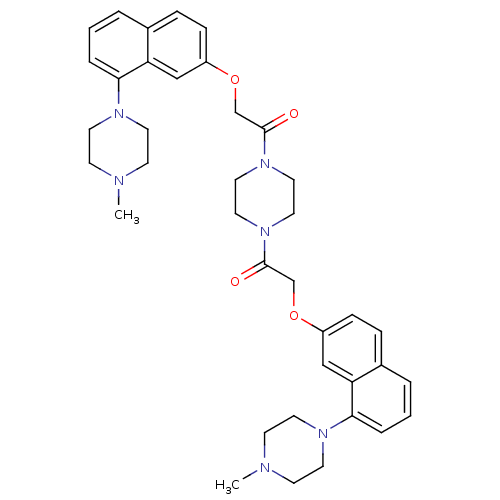 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070405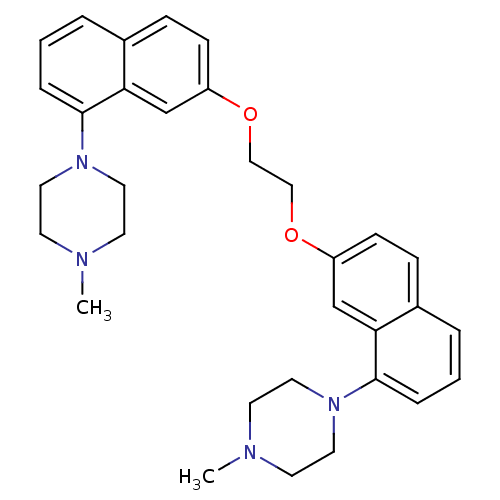 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070405 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070408 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50070407 (2N,2N-dipropyl-8-[6-(3-dipropylamino-1,2,3,4-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070408 (2-[8-(4-Methyl-piperazin-1-yl)-naphthalen-2-yloxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070406 (2-[3-(2-Amino-ethyl)-1H-indol-5-yloxy]-N-(4-{2-[8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50007407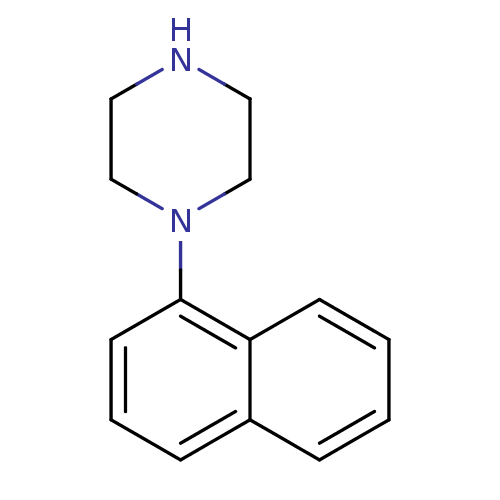 (1-NP | 1-Naphthalen-1-yl-piperazine | 4-Naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070407 (2N,2N-dipropyl-8-[6-(3-dipropylamino-1,2,3,4-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070407 (2N,2N-dipropyl-8-[6-(3-dipropylamino-1,2,3,4-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50007407 (1-NP | 1-Naphthalen-1-yl-piperazine | 4-Naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50007407 (1-NP | 1-Naphthalen-1-yl-piperazine | 4-Naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50070405 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1D receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 559 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070405 (1,2-di[8-(4-methylhexahydro-1-pyrazinyl)-2-naphthy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50007407 (1-NP | 1-Naphthalen-1-yl-piperazine | 4-Naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated at human recombinant 5-hydroxytryptamine 1B receptor using [3H]-5-CT as radioligand | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM21393 (7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50070407 (2N,2N-dipropyl-8-[6-(3-dipropylamino-1,2,3,4-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Centre de Recherche Pierre FABRE Curated by ChEMBL | Assay Description The intrinsic activity of the compound was evaluated for its ability to inhibit forskolin-stimulated c-AMP formation mediated by cloned 5-hydroxytryp... | Bioorg Med Chem Lett 8: 1407-12 (1999) BindingDB Entry DOI: 10.7270/Q2SQ8ZJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||