 Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50011434
Found 55 hits Enz. Inhib. hit(s) with all data for entry = 50011434 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106505

(6-Chloro-7-(indan-1-ylamino)-quinoline-5,8-dione |...)Show SMILES ClC1=C(NC2CCc3ccccc23)C(=O)c2ncccc2C1=O |c:1| Show InChI InChI=1S/C18H13ClN2O2/c19-14-16(18(23)15-12(17(14)22)6-3-9-20-15)21-13-8-7-10-4-1-2-5-11(10)13/h1-6,9,13,21H,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106491

(5-Amino-6-(7-amino-6-methoxy-5,8-dioxo-5,8-dihydro...)Show SMILES COC1C(=N)C(=O)c2nc(ccc2C1=O)-c1nc(C(=O)OC)c(C)c(c1N)-c1ccc(OC)c(OC)c1O |(10.32,-.31,;10.33,-1.86,;11.68,-2.63,;11.68,-4.17,;10.33,-4.94,;13,-4.93,;13.01,-6.48,;14.33,-4.16,;15.67,-4.93,;17,-4.16,;16.98,-2.62,;15.65,-1.86,;14.33,-2.63,;13,-1.85,;13,-.29,;18.33,-4.93,;19.66,-4.15,;21,-4.92,;22.33,-4.15,;22.31,-2.6,;23.67,-4.91,;25,-4.12,;21,-6.46,;22.34,-7.23,;19.66,-7.25,;18.33,-6.48,;17,-7.25,;19.67,-8.79,;18.33,-9.55,;18.33,-11.09,;19.66,-11.86,;19.67,-13.41,;18.33,-14.18,;21,-11.09,;22.34,-11.85,;22.35,-13.4,;21,-9.55,;22.33,-8.78,)| Show InChI InChI=1S/C26H24N4O8/c1-10-15(11-7-9-14(35-2)24(36-3)21(11)31)16(27)20(30-18(10)26(34)38-5)13-8-6-12-19(29-13)23(33)17(28)25(37-4)22(12)32/h6-9,25,28,31H,27H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106503
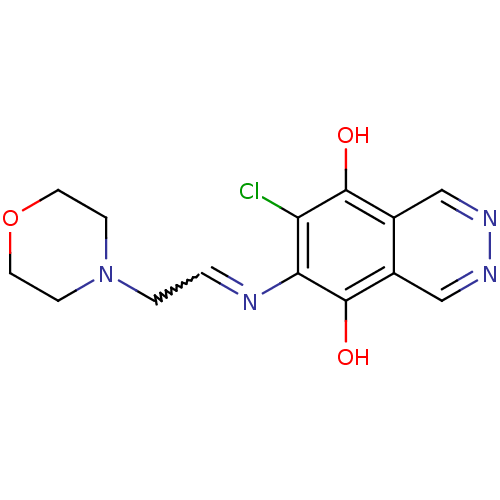
(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-phthalazi...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnncc12 |w:6.6| Show InChI InChI=1S/C14H15ClN4O3/c15-11-12(16-1-2-19-3-5-22-6-4-19)14(21)10-8-18-17-7-9(10)13(11)20/h1,7-8,20-21H,2-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106493

(7-[2-(4-Hydroxy-3-methoxy-phenyl)-ethylamino]-quin...)Show SMILES COc1cc(CC=Nc2cc(O)c3cccnc3c2O)ccc1O |w:6.5| Show InChI InChI=1S/C18H16N2O4/c1-24-16-9-11(4-5-14(16)21)6-8-19-13-10-15(22)12-3-2-7-20-17(12)18(13)23/h2-5,7-10,21-23H,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM80759
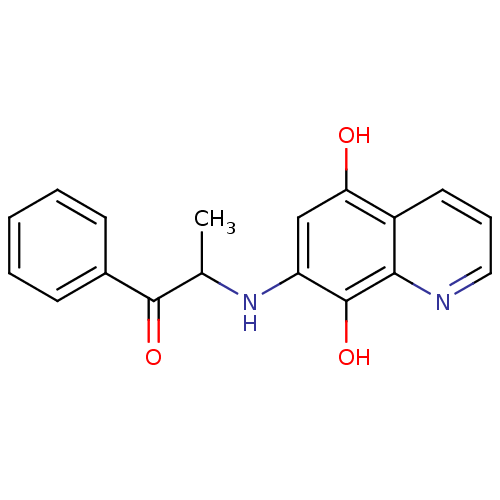
(7-[(1-hydroxy-1-phenylpropan-2-yl)amino]quinoline-...)Show InChI InChI=1S/C18H16N2O3/c1-11(17(22)12-6-3-2-4-7-12)20-14-10-15(21)13-8-5-9-19-16(13)18(14)23/h2-11,20-21,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106498
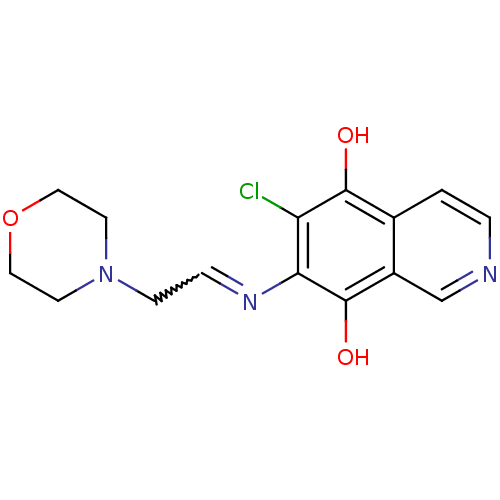
(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-isoquinol...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-12-13(18-3-4-19-5-7-22-8-6-19)15(21)11-9-17-2-1-10(11)14(12)20/h1-3,9,20-21H,4-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106508

(7-[2-(3,5-Dibromo-4-hydroxy-phenyl)-ethylamino]-qu...)Show SMILES Oc1cc(N=CCc2cc(Br)c(O)c(Br)c2)c(O)c2ncccc12 |w:5.5| Show InChI InChI=1S/C17H12Br2N2O3/c18-11-6-9(7-12(19)16(11)23)3-5-20-13-8-14(22)10-2-1-4-21-15(10)17(13)24/h1-2,4-8,22-24H,3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106495

(6-(2-Hydroxy-1-methyl-2-phenyl-ethylamino)-quinoli...)Show InChI InChI=1S/C18H16N2O3/c1-11(17(22)12-6-3-2-4-7-12)20-14-10-15(21)16-13(18(14)23)8-5-9-19-16/h2-11,20-21,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106494
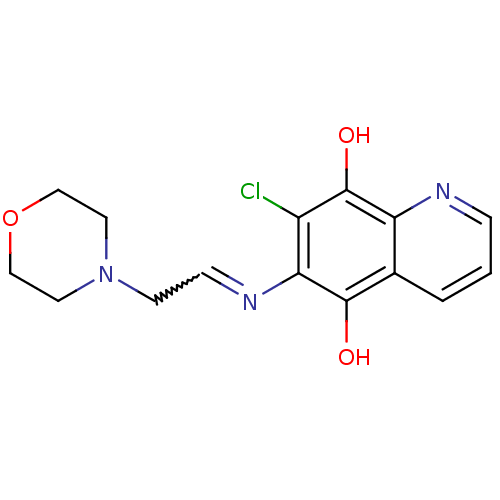
(7-Chloro-6-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cccnc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)14(20)10-2-1-3-17-12(10)15(11)21/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106506
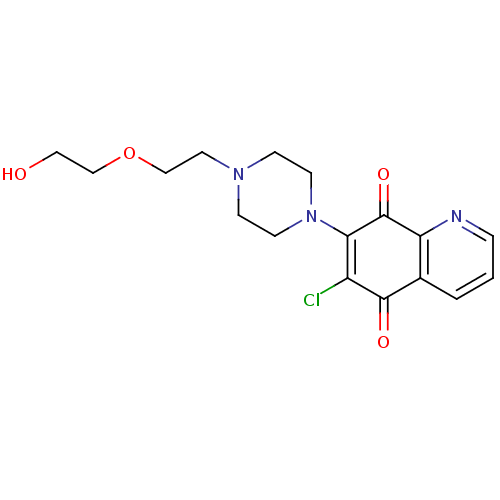
(6-Chloro-7-{4-[2-(2-hydroxy-ethoxy)-ethyl]-piperaz...)Show SMILES OCCOCCN1CCN(CC1)C1=C(Cl)C(=O)c2cccnc2C1=O |c:13| Show InChI InChI=1S/C17H20ClN3O4/c18-13-15(17(24)14-12(16(13)23)2-1-3-19-14)21-6-4-20(5-7-21)8-10-25-11-9-22/h1-3,22H,4-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106502

(6-Chloro-7-(4-hexyloxy-phenylamino)-quinoline-5,8-...)Show SMILES CCCCCCOc1ccc(NC2=C(Cl)C(=O)c3cccnc3C2=O)cc1 |c:12| Show InChI InChI=1S/C21H21ClN2O3/c1-2-3-4-5-13-27-15-10-8-14(9-11-15)24-19-17(22)20(25)16-7-6-12-23-18(16)21(19)26/h6-12,24H,2-5,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106503

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-phthalazi...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnncc12 |w:6.6| Show InChI InChI=1S/C14H15ClN4O3/c15-11-12(16-1-2-19-3-5-22-6-4-19)14(21)10-8-18-17-7-9(10)13(11)20/h1,7-8,20-21H,2-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106500
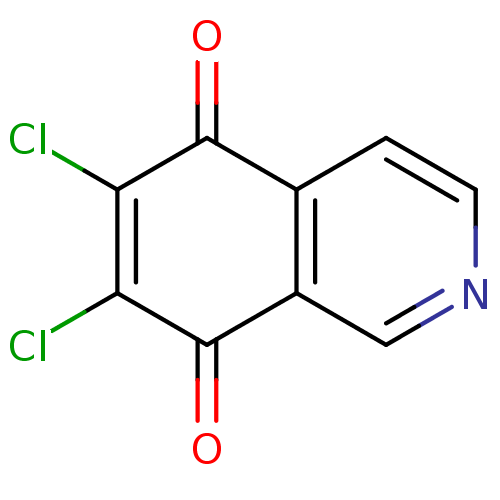
(6,7-Dichloro-isoquinoline-5,8-dione | CHEMBL335110)Show InChI InChI=1S/C9H3Cl2NO2/c10-6-7(11)9(14)5-3-12-2-1-4(5)8(6)13/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50080854
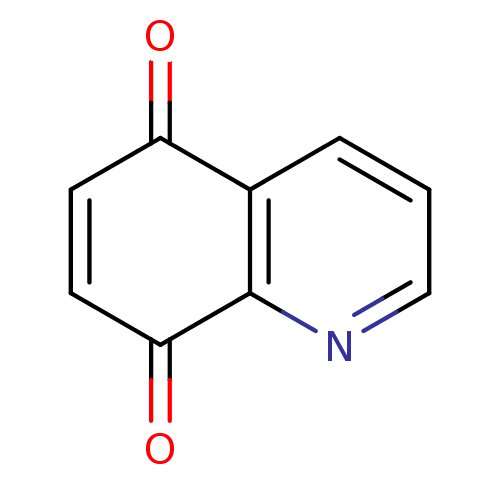
(CHEMBL310185 | Quinoline-5,8-dione)Show InChI InChI=1S/C9H5NO2/c11-7-3-4-8(12)9-6(7)2-1-5-10-9/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106497

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)15(21)12-10(14(11)20)2-1-3-17-12/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106501
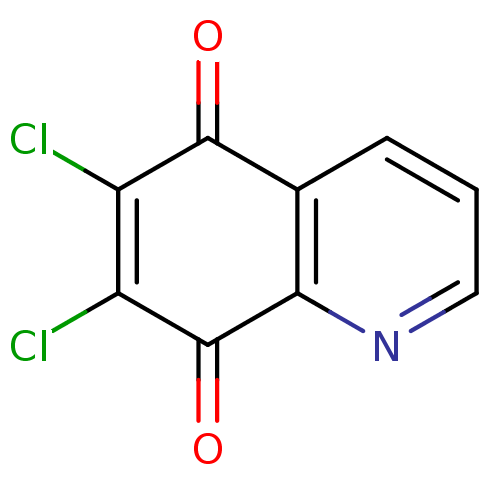
(6,7-Dichloro-5,8-quinolinequinone | 6,7-Dichloro-q...)Show InChI InChI=1S/C9H3Cl2NO2/c10-5-6(11)9(14)7-4(8(5)13)2-1-3-12-7/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106507
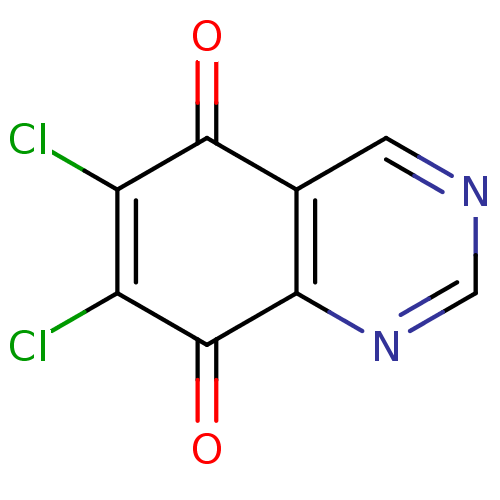
(6,7-Dichloro-quinazoline-5,8-dione | CHEMBL128764)Show InChI InChI=1S/C8H2Cl2N2O2/c9-4-5(10)8(14)6-3(7(4)13)1-11-2-12-6/h1-2H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106491

(5-Amino-6-(7-amino-6-methoxy-5,8-dioxo-5,8-dihydro...)Show SMILES COC1C(=N)C(=O)c2nc(ccc2C1=O)-c1nc(C(=O)OC)c(C)c(c1N)-c1ccc(OC)c(OC)c1O |(10.32,-.31,;10.33,-1.86,;11.68,-2.63,;11.68,-4.17,;10.33,-4.94,;13,-4.93,;13.01,-6.48,;14.33,-4.16,;15.67,-4.93,;17,-4.16,;16.98,-2.62,;15.65,-1.86,;14.33,-2.63,;13,-1.85,;13,-.29,;18.33,-4.93,;19.66,-4.15,;21,-4.92,;22.33,-4.15,;22.31,-2.6,;23.67,-4.91,;25,-4.12,;21,-6.46,;22.34,-7.23,;19.66,-7.25,;18.33,-6.48,;17,-7.25,;19.67,-8.79,;18.33,-9.55,;18.33,-11.09,;19.66,-11.86,;19.67,-13.41,;18.33,-14.18,;21,-11.09,;22.34,-11.85,;22.35,-13.4,;21,-9.55,;22.33,-8.78,)| Show InChI InChI=1S/C26H24N4O8/c1-10-15(11-7-9-14(35-2)24(36-3)21(11)31)16(27)20(30-18(10)26(34)38-5)13-8-6-12-19(29-13)23(33)17(28)25(37-4)22(12)32/h6-9,25,28,31H,27H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106499
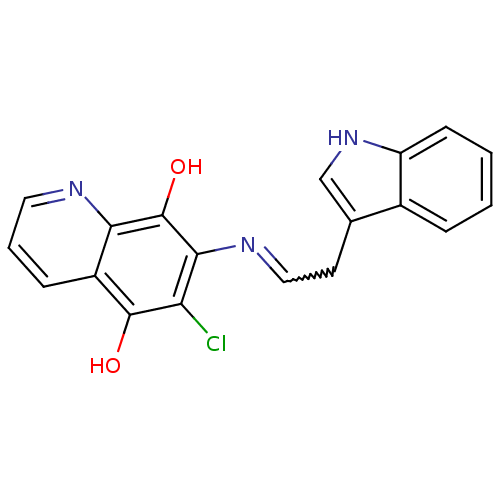
(6-Chloro-7-[2-(1H-indol-3-yl)-ethylamino]-quinolin...)Show SMILES Oc1c(Cl)c(N=CCc2c[nH]c3ccccc23)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C19H14ClN3O2/c20-15-17(19(25)16-13(18(15)24)5-3-8-21-16)22-9-7-11-10-23-14-6-2-1-4-12(11)14/h1-6,8-10,23-25H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106508

(7-[2-(3,5-Dibromo-4-hydroxy-phenyl)-ethylamino]-qu...)Show SMILES Oc1cc(N=CCc2cc(Br)c(O)c(Br)c2)c(O)c2ncccc12 |w:5.5| Show InChI InChI=1S/C17H12Br2N2O3/c18-11-6-9(7-12(19)16(11)23)3-5-20-13-8-14(22)10-2-1-4-21-15(10)17(13)24/h1-2,4-8,22-24H,3H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106508

(7-[2-(3,5-Dibromo-4-hydroxy-phenyl)-ethylamino]-qu...)Show SMILES Oc1cc(N=CCc2cc(Br)c(O)c(Br)c2)c(O)c2ncccc12 |w:5.5| Show InChI InChI=1S/C17H12Br2N2O3/c18-11-6-9(7-12(19)16(11)23)3-5-20-13-8-14(22)10-2-1-4-21-15(10)17(13)24/h1-2,4-8,22-24H,3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106491

(5-Amino-6-(7-amino-6-methoxy-5,8-dioxo-5,8-dihydro...)Show SMILES COC1C(=N)C(=O)c2nc(ccc2C1=O)-c1nc(C(=O)OC)c(C)c(c1N)-c1ccc(OC)c(OC)c1O |(10.32,-.31,;10.33,-1.86,;11.68,-2.63,;11.68,-4.17,;10.33,-4.94,;13,-4.93,;13.01,-6.48,;14.33,-4.16,;15.67,-4.93,;17,-4.16,;16.98,-2.62,;15.65,-1.86,;14.33,-2.63,;13,-1.85,;13,-.29,;18.33,-4.93,;19.66,-4.15,;21,-4.92,;22.33,-4.15,;22.31,-2.6,;23.67,-4.91,;25,-4.12,;21,-6.46,;22.34,-7.23,;19.66,-7.25,;18.33,-6.48,;17,-7.25,;19.67,-8.79,;18.33,-9.55,;18.33,-11.09,;19.66,-11.86,;19.67,-13.41,;18.33,-14.18,;21,-11.09,;22.34,-11.85,;22.35,-13.4,;21,-9.55,;22.33,-8.78,)| Show InChI InChI=1S/C26H24N4O8/c1-10-15(11-7-9-14(35-2)24(36-3)21(11)31)16(27)20(30-18(10)26(34)38-5)13-8-6-12-19(29-13)23(33)17(28)25(37-4)22(12)32/h6-9,25,28,31H,27H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106496
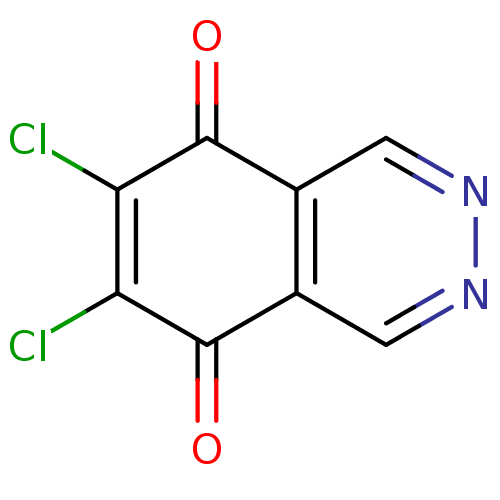
(6,7-Dichloro-phthalazine-5,8-dione | CHEMBL132324)Show InChI InChI=1S/C8H2Cl2N2O2/c9-5-6(10)8(14)4-2-12-11-1-3(4)7(5)13/h1-2H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106503

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-phthalazi...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnncc12 |w:6.6| Show InChI InChI=1S/C14H15ClN4O3/c15-11-12(16-1-2-19-3-5-22-6-4-19)14(21)10-8-18-17-7-9(10)13(11)20/h1,7-8,20-21H,2-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106506

(6-Chloro-7-{4-[2-(2-hydroxy-ethoxy)-ethyl]-piperaz...)Show SMILES OCCOCCN1CCN(CC1)C1=C(Cl)C(=O)c2cccnc2C1=O |c:13| Show InChI InChI=1S/C17H20ClN3O4/c18-13-15(17(24)14-12(16(13)23)2-1-3-19-14)21-6-4-20(5-7-21)8-10-25-11-9-22/h1-3,22H,4-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106501

(6,7-Dichloro-5,8-quinolinequinone | 6,7-Dichloro-q...)Show InChI InChI=1S/C9H3Cl2NO2/c10-5-6(11)9(14)7-4(8(5)13)2-1-3-12-7/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106507

(6,7-Dichloro-quinazoline-5,8-dione | CHEMBL128764)Show InChI InChI=1S/C8H2Cl2N2O2/c9-4-5(10)8(14)6-3(7(4)13)1-11-2-12-6/h1-2H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50080854

(CHEMBL310185 | Quinoline-5,8-dione)Show InChI InChI=1S/C9H5NO2/c11-7-3-4-8(12)9-6(7)2-1-5-10-9/h1-5H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106500

(6,7-Dichloro-isoquinoline-5,8-dione | CHEMBL335110)Show InChI InChI=1S/C9H3Cl2NO2/c10-6-7(11)9(14)5-3-12-2-1-4(5)8(6)13/h1-3H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106499

(6-Chloro-7-[2-(1H-indol-3-yl)-ethylamino]-quinolin...)Show SMILES Oc1c(Cl)c(N=CCc2c[nH]c3ccccc23)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C19H14ClN3O2/c20-15-17(19(25)16-13(18(15)24)5-3-8-21-16)22-9-7-11-10-23-14-6-2-1-4-12(11)14/h1-6,8-10,23-25H,7H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106507

(6,7-Dichloro-quinazoline-5,8-dione | CHEMBL128764)Show InChI InChI=1S/C8H2Cl2N2O2/c9-4-5(10)8(14)6-3(7(4)13)1-11-2-12-6/h1-2H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106506

(6-Chloro-7-{4-[2-(2-hydroxy-ethoxy)-ethyl]-piperaz...)Show SMILES OCCOCCN1CCN(CC1)C1=C(Cl)C(=O)c2cccnc2C1=O |c:13| Show InChI InChI=1S/C17H20ClN3O4/c18-13-15(17(24)14-12(16(13)23)2-1-3-19-14)21-6-4-20(5-7-21)8-10-25-11-9-22/h1-3,22H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106502

(6-Chloro-7-(4-hexyloxy-phenylamino)-quinoline-5,8-...)Show SMILES CCCCCCOc1ccc(NC2=C(Cl)C(=O)c3cccnc3C2=O)cc1 |c:12| Show InChI InChI=1S/C21H21ClN2O3/c1-2-3-4-5-13-27-15-10-8-14(9-11-15)24-19-17(22)20(25)16-7-6-12-23-18(16)21(19)26/h6-12,24H,2-5,13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106495

(6-(2-Hydroxy-1-methyl-2-phenyl-ethylamino)-quinoli...)Show InChI InChI=1S/C18H16N2O3/c1-11(17(22)12-6-3-2-4-7-12)20-14-10-15(21)16-13(18(14)23)8-5-9-19-16/h2-11,20-21,23H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106499

(6-Chloro-7-[2-(1H-indol-3-yl)-ethylamino]-quinolin...)Show SMILES Oc1c(Cl)c(N=CCc2c[nH]c3ccccc23)c(O)c2ncccc12 |w:6.6| Show InChI InChI=1S/C19H14ClN3O2/c20-15-17(19(25)16-13(18(15)24)5-3-8-21-16)22-9-7-11-10-23-14-6-2-1-4-12(11)14/h1-6,8-10,23-25H,7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50080854

(CHEMBL310185 | Quinoline-5,8-dione)Show InChI InChI=1S/C9H5NO2/c11-7-3-4-8(12)9-6(7)2-1-5-10-9/h1-5H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106501

(6,7-Dichloro-5,8-quinolinequinone | 6,7-Dichloro-q...)Show InChI InChI=1S/C9H3Cl2NO2/c10-5-6(11)9(14)7-4(8(5)13)2-1-3-12-7/h1-3H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106502

(6-Chloro-7-(4-hexyloxy-phenylamino)-quinoline-5,8-...)Show SMILES CCCCCCOc1ccc(NC2=C(Cl)C(=O)c3cccnc3C2=O)cc1 |c:12| Show InChI InChI=1S/C21H21ClN2O3/c1-2-3-4-5-13-27-15-10-8-14(9-11-15)24-19-17(22)20(25)16-7-6-12-23-18(16)21(19)26/h6-12,24H,2-5,13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106500

(6,7-Dichloro-isoquinoline-5,8-dione | CHEMBL335110)Show InChI InChI=1S/C9H3Cl2NO2/c10-6-7(11)9(14)5-3-12-2-1-4(5)8(6)13/h1-3H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106496

(6,7-Dichloro-phthalazine-5,8-dione | CHEMBL132324)Show InChI InChI=1S/C8H2Cl2N2O2/c9-5-6(10)8(14)4-2-12-11-1-3(4)7(5)13/h1-2H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106498

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-isoquinol...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-12-13(18-3-4-19-5-7-22-8-6-19)15(21)11-9-17-2-1-10(11)14(12)20/h1-3,9,20-21H,4-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106498

(6-Chloro-7-(2-morpholin-4-yl-ethylamino)-isoquinol...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cnccc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-12-13(18-3-4-19-5-7-22-8-6-19)15(21)11-9-17-2-1-10(11)14(12)20/h1-3,9,20-21H,4-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106496

(6,7-Dichloro-phthalazine-5,8-dione | CHEMBL132324)Show InChI InChI=1S/C8H2Cl2N2O2/c9-5-6(10)8(14)4-2-12-11-1-3(4)7(5)13/h1-2H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106495

(6-(2-Hydroxy-1-methyl-2-phenyl-ethylamino)-quinoli...)Show InChI InChI=1S/C18H16N2O3/c1-11(17(22)12-6-3-2-4-7-12)20-14-10-15(21)16-13(18(14)23)8-5-9-19-16/h2-11,20-21,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106494

(7-Chloro-6-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cccnc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)14(20)10-2-1-3-17-12(10)15(11)21/h1-4,20-21H,5-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50106492
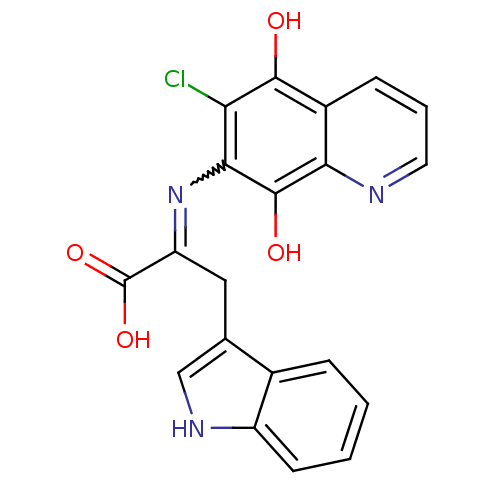
(2-(6-Chloro-5,8-dioxo-5,8-dihydro-quinolin-7-ylami...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)=Nc1c(O)c2ncccc2c(O)c1Cl |w:14.16| Show InChI InChI=1S/C20H14ClN3O4/c21-15-17(19(26)16-12(18(15)25)5-3-7-22-16)24-14(20(27)28)8-10-9-23-13-6-2-1-4-11(10)13/h1-7,9,23,25-26H,8H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human VHR |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106492

(2-(6-Chloro-5,8-dioxo-5,8-dihydro-quinolin-7-ylami...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)=Nc1c(O)c2ncccc2c(O)c1Cl |w:14.16| Show InChI InChI=1S/C20H14ClN3O4/c21-15-17(19(26)16-12(18(15)25)5-3-7-22-16)24-14(20(27)28)8-10-9-23-13-6-2-1-4-11(10)13/h1-7,9,23,25-26H,8H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50106494

(7-Chloro-6-(2-morpholin-4-yl-ethylamino)-quinoline...)Show SMILES Oc1c(Cl)c(N=CCN2CCOCC2)c(O)c2cccnc12 |w:6.6| Show InChI InChI=1S/C15H16ClN3O3/c16-11-13(18-4-5-19-6-8-22-9-7-19)14(20)10-2-1-3-17-12(10)15(11)21/h1-4,20-21H,5-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory Activity against Recombinant Human PTP1 |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50106492

(2-(6-Chloro-5,8-dioxo-5,8-dihydro-quinolin-7-ylami...)Show SMILES OC(=O)C(Cc1c[nH]c2ccccc12)=Nc1c(O)c2ncccc2c(O)c1Cl |w:14.16| Show InChI InChI=1S/C20H14ClN3O4/c21-15-17(19(26)16-12(18(15)25)5-3-7-22-16)24-14(20(27)28)8-10-9-23-13-6-2-1-4-11(10)13/h1-7,9,23,25-26H,8H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human cell division cycle 25B |
J Med Chem 44: 4042-9 (2001)
BindingDB Entry DOI: 10.7270/Q2X34WR5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data