 Found 65 hits Enz. Inhib. hit(s) with all data for entry = 50012135
Found 65 hits Enz. Inhib. hit(s) with all data for entry = 50012135 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115163
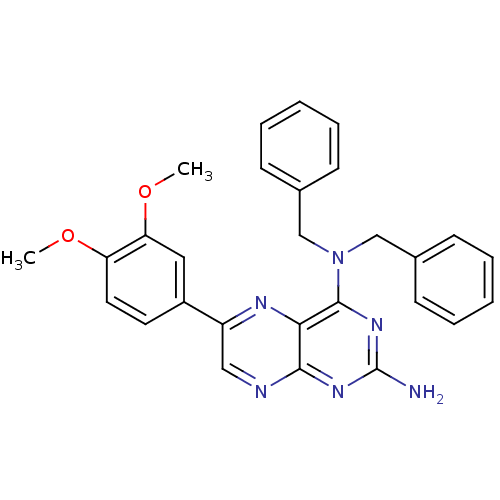
(CHEMBL101062 | N*4*,N*4*-Dibenzyl-6-(3,4-dimethoxy...)Show SMILES COc1ccc(cc1OC)-c1cnc2nc(N)nc(N(Cc3ccccc3)Cc3ccccc3)c2n1 Show InChI InChI=1S/C28H26N6O2/c1-35-23-14-13-21(15-24(23)36-2)22-16-30-26-25(31-22)27(33-28(29)32-26)34(17-19-9-5-3-6-10-19)18-20-11-7-4-8-12-20/h3-16H,17-18H2,1-2H3,(H2,29,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081589
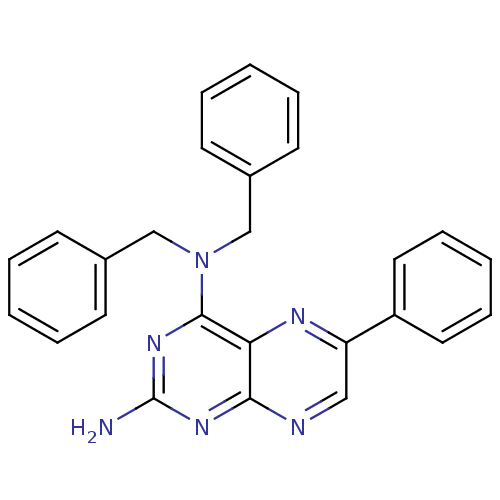
(CHEMBL101399 | N*4*,N*4*-Dibenzyl-6-phenyl-pteridi...)Show SMILES Nc1nc(N(Cc2ccccc2)Cc2ccccc2)c2nc(cnc2n1)-c1ccccc1 Show InChI InChI=1S/C26H22N6/c27-26-30-24-23(29-22(16-28-24)21-14-8-3-9-15-21)25(31-26)32(17-19-10-4-1-5-11-19)18-20-12-6-2-7-13-20/h1-16H,17-18H2,(H2,27,28,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081585
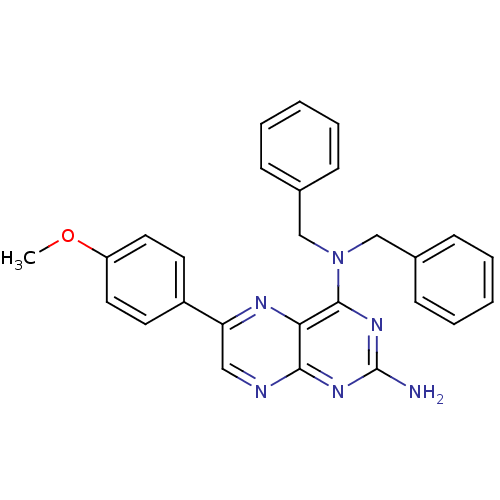
(CHEMBL100659 | N*4*,N*4*-Dibenzyl-6-(4-methoxy-phe...)Show SMILES COc1ccc(cc1)-c1cnc2nc(N)nc(N(Cc3ccccc3)Cc3ccccc3)c2n1 Show InChI InChI=1S/C27H24N6O/c1-34-22-14-12-21(13-15-22)23-16-29-25-24(30-23)26(32-27(28)31-25)33(17-19-8-4-2-5-9-19)18-20-10-6-3-7-11-20/h2-16H,17-18H2,1H3,(H2,28,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115131
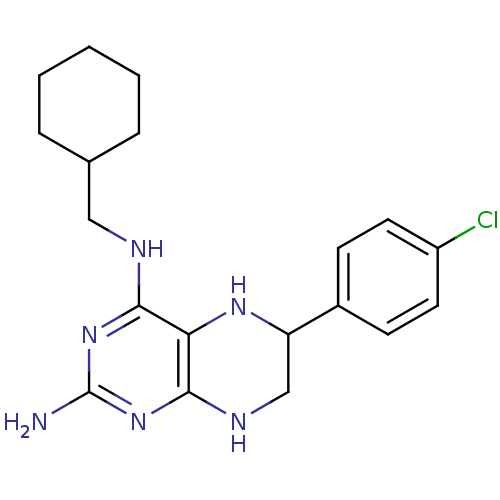
(6-(4-Chloro-phenyl)-N*4*-cyclohexylmethyl-5,6,7,8-...)Show InChI InChI=1S/C19H25ClN6/c20-14-8-6-13(7-9-14)15-11-23-18-16(24-15)17(25-19(21)26-18)22-10-12-4-2-1-3-5-12/h6-9,12,15,24H,1-5,10-11H2,(H4,21,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115151
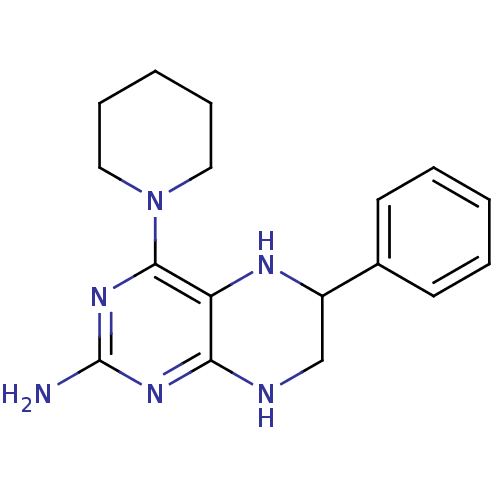
(6-Phenyl-4-piperidin-1-yl-5,6,7,8-tetrahydro-pteri...)Show InChI InChI=1S/C17H22N6/c18-17-21-15-14(16(22-17)23-9-5-2-6-10-23)20-13(11-19-15)12-7-3-1-4-8-12/h1,3-4,7-8,13,20H,2,5-6,9-11H2,(H3,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081594
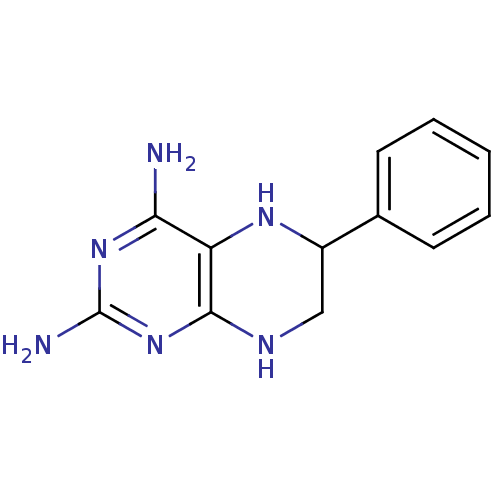
(6-Phenyl-5,6,7,8-tetrahydro-pteridine-2,4-diamine ...)Show InChI InChI=1S/C12H14N6/c13-10-9-11(18-12(14)17-10)15-6-8(16-9)7-4-2-1-3-5-7/h1-5,8,16H,6H2,(H5,13,14,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115138
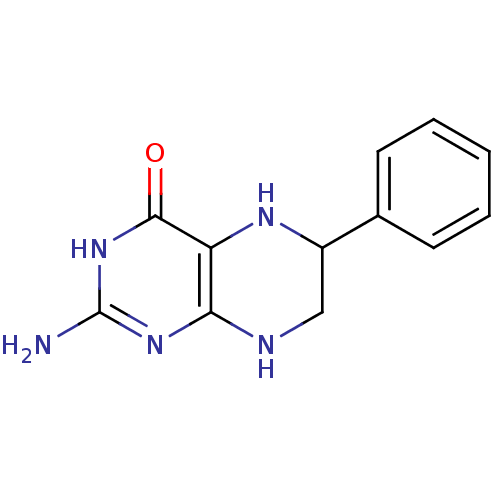
(2-Amino-6-phenyl-5,6,7,8-tetrahydro-3H-pteridin-4-...)Show InChI InChI=1S/C12H13N5O/c13-12-16-10-9(11(18)17-12)15-8(6-14-10)7-4-2-1-3-5-7/h1-5,8,15H,6H2,(H4,13,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115165

(4-Pentyloxy-6-phenylethynyl-pteridin-2-ylamine | C...)Show InChI InChI=1S/C19H19N5O/c1-2-3-7-12-25-18-16-17(23-19(20)24-18)21-13-15(22-16)11-10-14-8-5-4-6-9-14/h4-6,8-9,13H,2-3,7,12H2,1H3,(H2,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115154
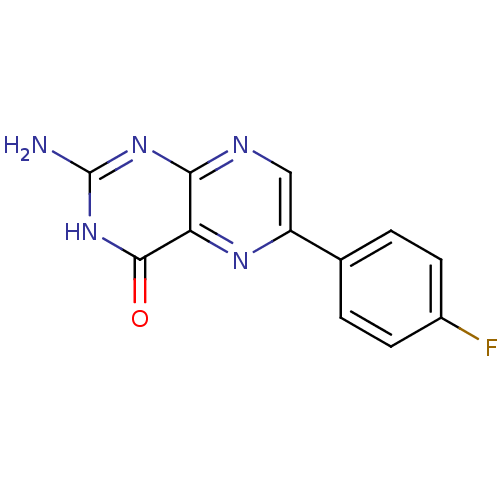
(2-Amino-6-(4-fluoro-phenyl)-3H-pteridin-4-one | CH...)Show InChI InChI=1S/C12H8FN5O/c13-7-3-1-6(2-4-7)8-5-15-10-9(16-8)11(19)18-12(14)17-10/h1-5H,(H3,14,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115159
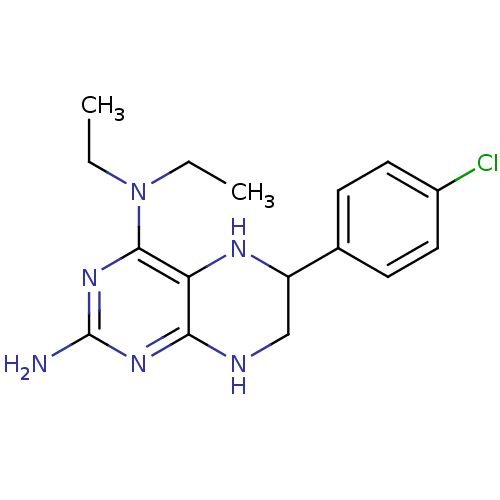
(6-(4-Chloro-phenyl)-N*4*,N*4*-diethyl-5,6,7,8-tetr...)Show InChI InChI=1S/C16H21ClN6/c1-3-23(4-2)15-13-14(21-16(18)22-15)19-9-12(20-13)10-5-7-11(17)8-6-10/h5-8,12,20H,3-4,9H2,1-2H3,(H3,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115141

(2-Amino-6-naphthalen-2-yl-3H-pteridin-4-one | CHEM...)Show InChI InChI=1S/C16H11N5O/c17-16-20-14-13(15(22)21-16)19-12(8-18-14)11-6-5-9-3-1-2-4-10(9)7-11/h1-8H,(H3,17,18,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081588
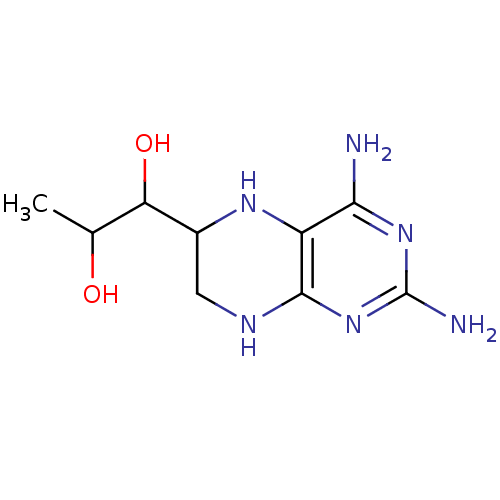
(1-(2,4-Diamino-5,6,7,8-tetrahydro-pteridin-6-yl)-p...)Show InChI InChI=1S/C9H16N6O2/c1-3(16)6(17)4-2-12-8-5(13-4)7(10)14-9(11)15-8/h3-4,6,13,16-17H,2H2,1H3,(H5,10,11,12,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115135
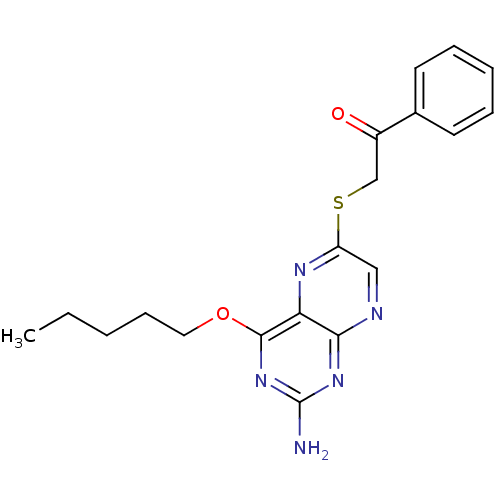
(2-(2-Amino-4-pentyloxy-pteridin-6-ylsulfanyl)-1-ph...)Show InChI InChI=1S/C19H21N5O2S/c1-2-3-7-10-26-18-16-17(23-19(20)24-18)21-11-15(22-16)27-12-14(25)13-8-5-4-6-9-13/h4-6,8-9,11H,2-3,7,10,12H2,1H3,(H2,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115171
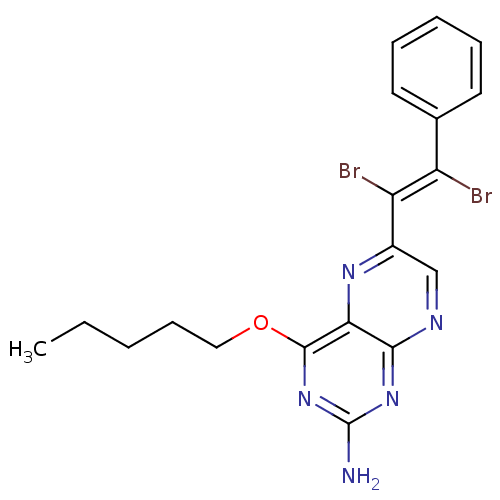
(6-(1,2-Dibromo-2-phenyl-vinyl)-4-pentyloxy-pteridi...)Show SMILES CCCCCOc1nc(N)nc2ncc(nc12)C(\Br)=C(/Br)c1ccccc1 Show InChI InChI=1S/C19H19Br2N5O/c1-2-3-7-10-27-18-16-17(25-19(22)26-18)23-11-13(24-16)15(21)14(20)12-8-5-4-6-9-12/h4-6,8-9,11H,2-3,7,10H2,1H3,(H2,22,23,25,26)/b15-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115162
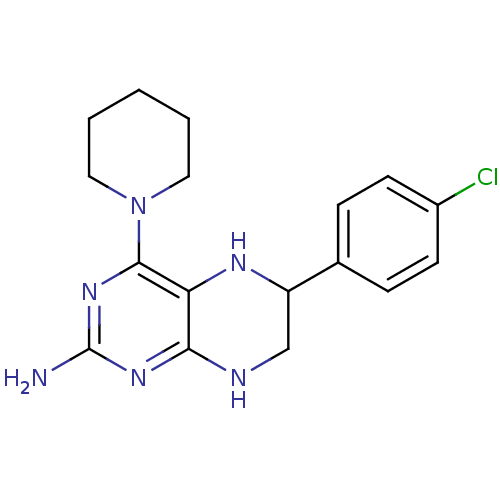
(6-(4-Chloro-phenyl)-4-piperidin-1-yl-5,6,7,8-tetra...)Show InChI InChI=1S/C17H21ClN6/c18-12-6-4-11(5-7-12)13-10-20-15-14(21-13)16(23-17(19)22-15)24-8-2-1-3-9-24/h4-7,13,21H,1-3,8-10H2,(H3,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115130
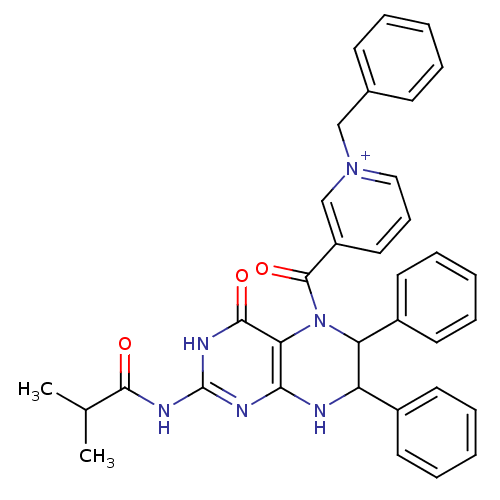
(1-Benzyl-3-(2-isobutyrylamino-4-oxo-6,7-diphenyl-4...)Show SMILES CC(C)C(=O)Nc1nc2NC(C(N(C(=O)c3ccc[n+](Cc4ccccc4)c3)c2c(=O)[nH]1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H32N6O3/c1-23(2)32(42)38-35-37-31-30(33(43)39-35)41(34(44)27-19-12-20-40(22-27)21-24-13-6-3-7-14-24)29(26-17-10-5-11-18-26)28(36-31)25-15-8-4-9-16-25/h3-20,22-23,28-29H,21H2,1-2H3,(H2-,36,37,38,39,42,43)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115150
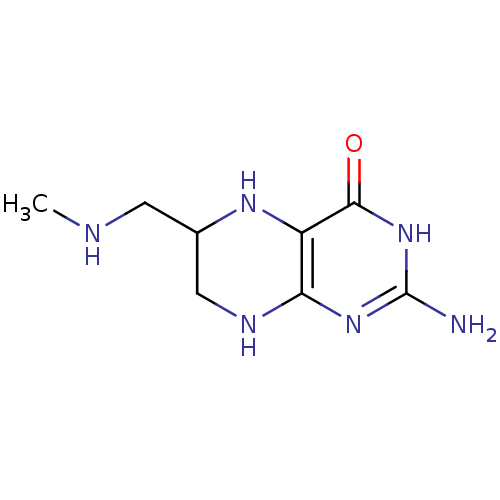
(2-Amino-6-methylaminomethyl-5,6,7,8-tetrahydro-3H-...)Show InChI InChI=1S/C8H14N6O/c1-10-2-4-3-11-6-5(12-4)7(15)14-8(9)13-6/h4,10,12H,2-3H2,1H3,(H4,9,11,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115128
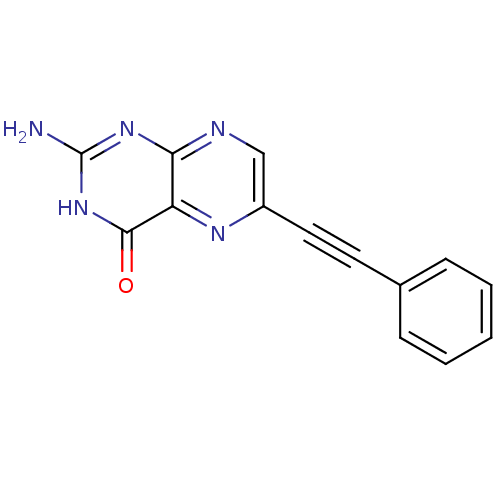
(2-Amino-6-phenylethynyl-3H-pteridin-4-one | CHEMBL...)Show InChI InChI=1S/C14H9N5O/c15-14-18-12-11(13(20)19-14)17-10(8-16-12)7-6-9-4-2-1-3-5-9/h1-5,8H,(H3,15,16,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081582
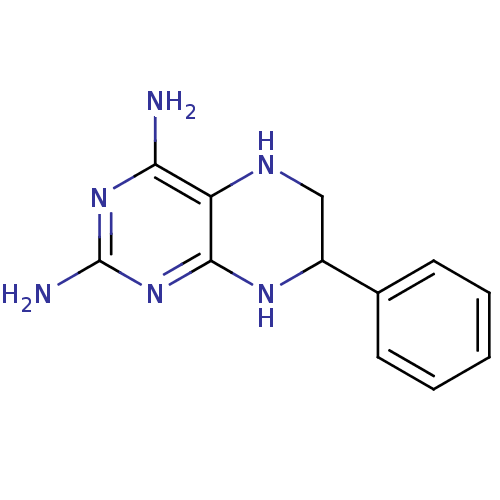
(7-Phenyl-5,6,7,8-tetrahydro-pteridine-2,4-diamine ...)Show InChI InChI=1S/C12H14N6/c13-10-9-11(18-12(14)17-10)16-8(6-15-9)7-4-2-1-3-5-7/h1-5,8,15H,6H2,(H5,13,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115152
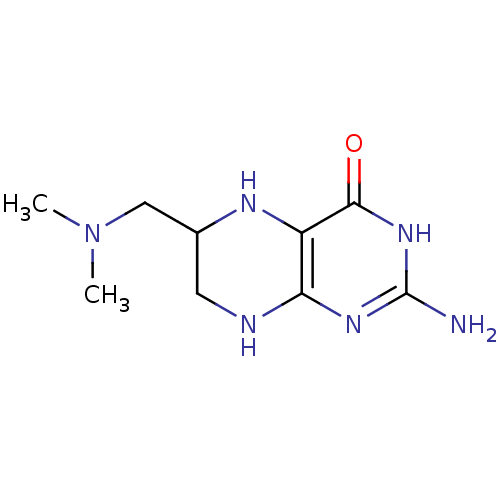
(2-Amino-6-dimethylaminomethyl-5,6,7,8-tetrahydro-3...)Show InChI InChI=1S/C9H16N6O/c1-15(2)4-5-3-11-7-6(12-5)8(16)14-9(10)13-7/h5,12H,3-4H2,1-2H3,(H4,10,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115161
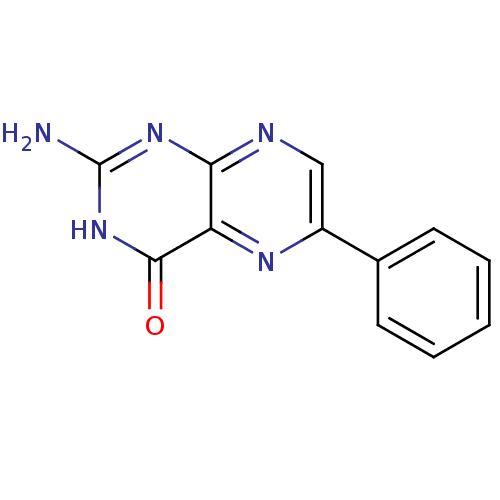
(2-Amino-6-phenyl-3H-pteridin-4-one | CHEMBL316947)Show InChI InChI=1S/C12H9N5O/c13-12-16-10-9(11(18)17-12)15-8(6-14-10)7-4-2-1-3-5-7/h1-6H,(H3,13,14,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115170
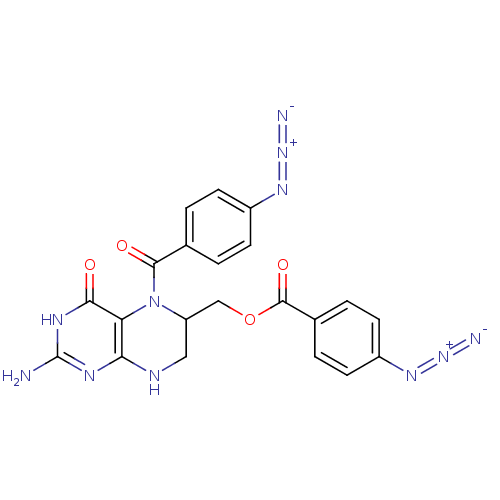
(4-Azido-benzoic acid 2-amino-5-(4-azido-benzoyl)-4...)Show SMILES Nc1nc2NCC(COC(=O)c3ccc(cc3)N=[N+]=[N-])N(C(=O)c3ccc(cc3)N=[N+]=[N-])c2c(=O)[nH]1 Show InChI InChI=1S/C21H17N11O4/c22-21-26-17-16(18(33)27-21)32(19(34)11-1-5-13(6-2-11)28-30-23)15(9-25-17)10-36-20(35)12-3-7-14(8-4-12)29-31-24/h1-8,15H,9-10H2,(H4,22,25,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115172
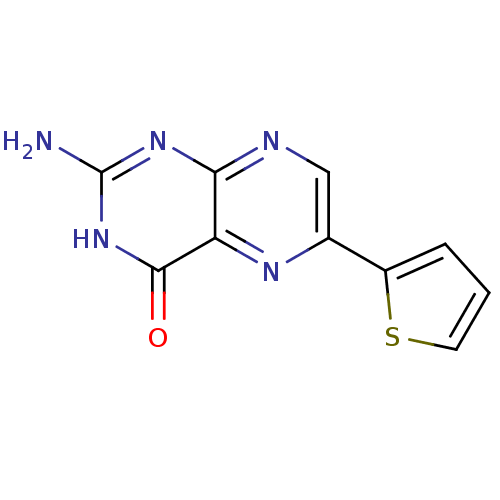
(2-Amino-6-thiophen-2-yl-3H-pteridin-4-one | CHEMBL...)Show InChI InChI=1S/C10H7N5OS/c11-10-14-8-7(9(16)15-10)13-5(4-12-8)6-2-1-3-17-6/h1-4H,(H3,11,12,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081583
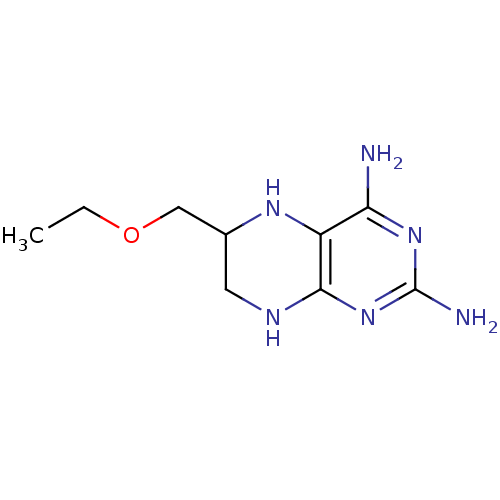
(6-Ethoxymethyl-5,6,7,8-tetrahydro-pteridine-2,4-di...)Show InChI InChI=1S/C9H16N6O/c1-2-16-4-5-3-12-8-6(13-5)7(10)14-9(11)15-8/h5,13H,2-4H2,1H3,(H5,10,11,12,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081587

(6-Decyloxymethyl-pteridine-2,4-diamine | CHEMBL986...)Show InChI InChI=1S/C17H28N6O/c1-2-3-4-5-6-7-8-9-10-24-12-13-11-20-16-14(21-13)15(18)22-17(19)23-16/h11H,2-10,12H2,1H3,(H4,18,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115158
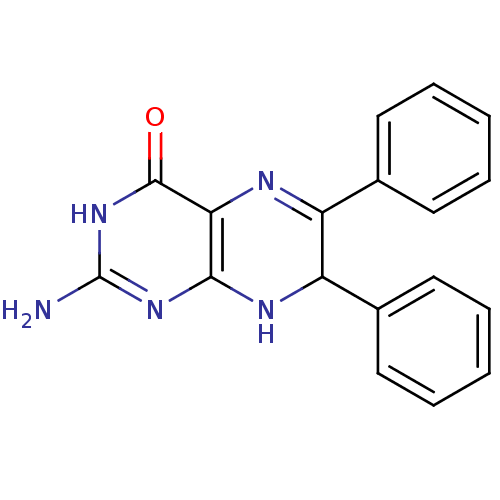
(2-Amino-6,7-diphenyl-7,8-dihydro-3H-pteridin-4-one...)Show SMILES Nc1nc2NC(C(=Nc2c(=O)[nH]1)c1ccccc1)c1ccccc1 |c:6| Show InChI InChI=1S/C18H15N5O/c19-18-22-16-15(17(24)23-18)20-13(11-7-3-1-4-8-11)14(21-16)12-9-5-2-6-10-12/h1-10,14H,(H4,19,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115142
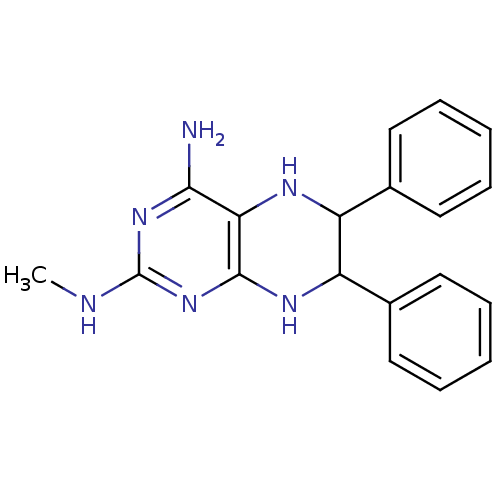
(CHEMBL99500 | N*2*-Methyl-6,7-diphenyl-5,6,7,8-tet...)Show InChI InChI=1S/C19H20N6/c1-21-19-24-17(20)16-18(25-19)23-15(13-10-6-3-7-11-13)14(22-16)12-8-4-2-5-9-12/h2-11,14-15,22H,1H3,(H4,20,21,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081586
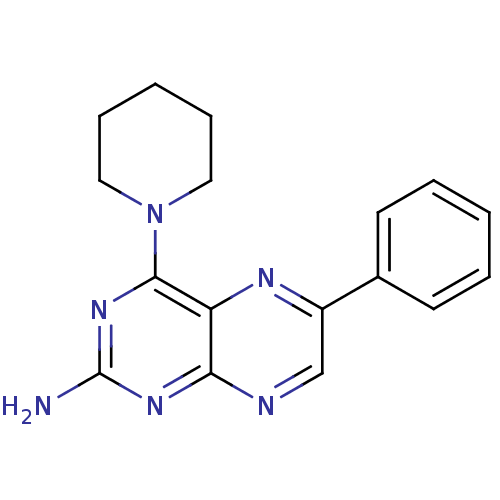
(6-Phenyl-4-piperidin-1-yl-pteridin-2-ylamine | CHE...)Show InChI InChI=1S/C17H18N6/c18-17-21-15-14(16(22-17)23-9-5-2-6-10-23)20-13(11-19-15)12-7-3-1-4-8-12/h1,3-4,7-8,11H,2,5-6,9-10H2,(H2,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115160
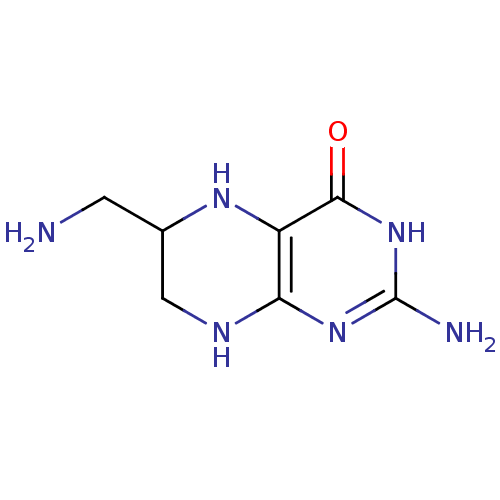
(2-Amino-6-aminomethyl-5,6,7,8-tetrahydro-3H-pterid...)Show InChI InChI=1S/C7H12N6O/c8-1-3-2-10-5-4(11-3)6(14)13-7(9)12-5/h3,11H,1-2,8H2,(H4,9,10,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081579
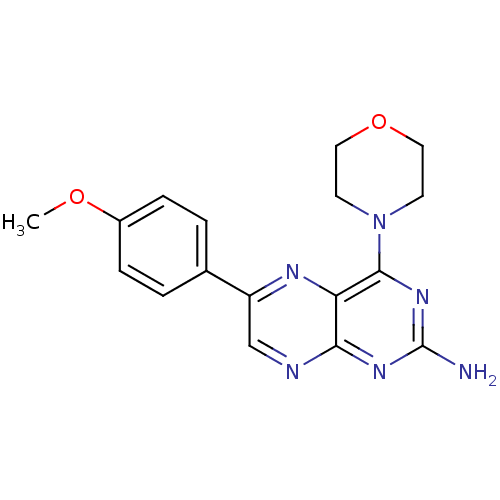
(6-(4-Methoxy-phenyl)-4-morpholin-4-yl-pteridin-2-y...)Show InChI InChI=1S/C17H18N6O2/c1-24-12-4-2-11(3-5-12)13-10-19-15-14(20-13)16(22-17(18)21-15)23-6-8-25-9-7-23/h2-5,10H,6-9H2,1H3,(H2,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115168
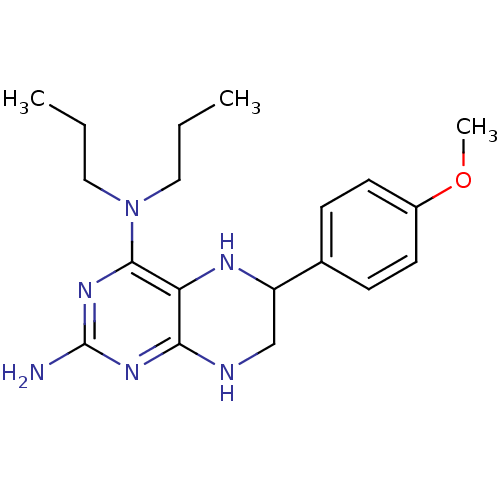
(6-(4-Methoxy-phenyl)-N*4*,N*4*-dipropyl-5,6,7,8-te...)Show InChI InChI=1S/C19H28N6O/c1-4-10-25(11-5-2)18-16-17(23-19(20)24-18)21-12-15(22-16)13-6-8-14(26-3)9-7-13/h6-9,15,22H,4-5,10-12H2,1-3H3,(H3,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115134
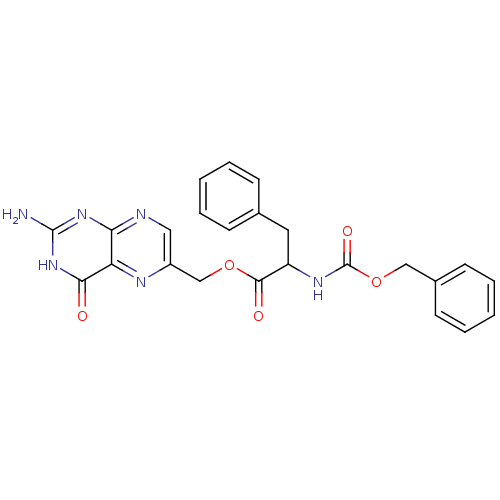
(2-Benzyloxycarbonylamino-3-phenyl-propionic acid 2...)Show SMILES Nc1nc2ncc(COC(=O)C(Cc3ccccc3)NC(=O)OCc3ccccc3)nc2c(=O)[nH]1 Show InChI InChI=1S/C24H22N6O5/c25-23-29-20-19(21(31)30-23)27-17(12-26-20)14-34-22(32)18(11-15-7-3-1-4-8-15)28-24(33)35-13-16-9-5-2-6-10-16/h1-10,12,18H,11,13-14H2,(H,28,33)(H3,25,26,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115146
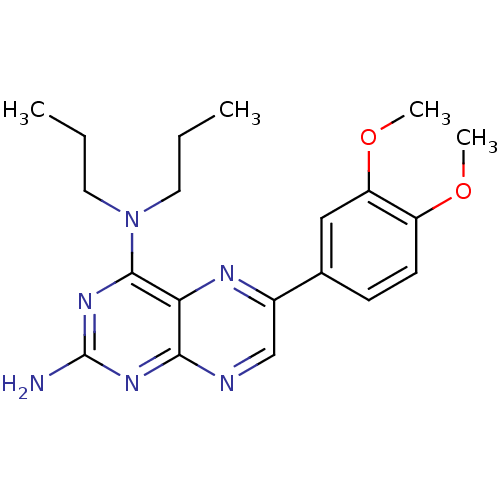
(6-(3,4-Dimethoxy-phenyl)-N*4*,N*4*-dipropyl-pterid...)Show SMILES CCCN(CCC)c1nc(N)nc2ncc(nc12)-c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C20H26N6O2/c1-5-9-26(10-6-2)19-17-18(24-20(21)25-19)22-12-14(23-17)13-7-8-15(27-3)16(11-13)28-4/h7-8,11-12H,5-6,9-10H2,1-4H3,(H2,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115155
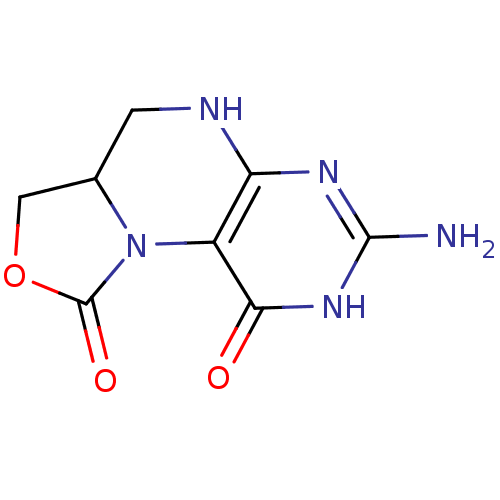
(7-Amino-3,3a,4,5-tetrahydro-8H-2-oxa-5,6,8,9b-tetr...)Show InChI InChI=1S/C8H9N5O3/c9-7-11-5-4(6(14)12-7)13-3(1-10-5)2-16-8(13)15/h3H,1-2H2,(H4,9,10,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115133
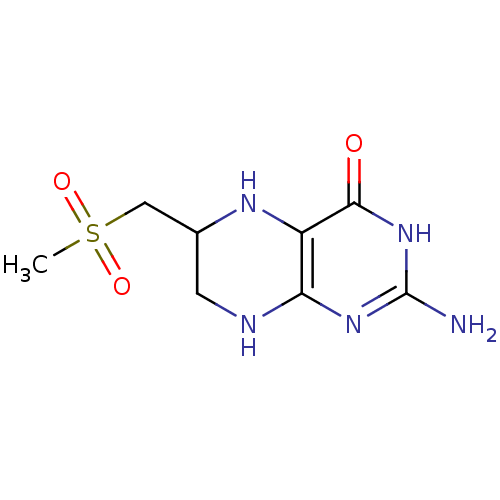
(2-Amino-6-methanesulfonylmethyl-5,6,7,8-tetrahydro...)Show InChI InChI=1S/C8H13N5O3S/c1-17(15,16)3-4-2-10-6-5(11-4)7(14)13-8(9)12-6/h4,11H,2-3H2,1H3,(H4,9,10,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081584
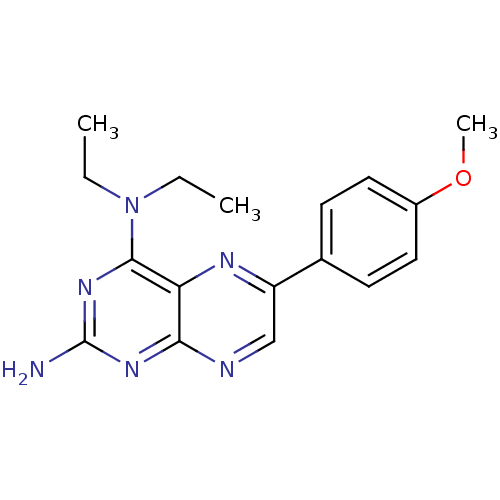
(CHEMBL100658 | N*4*,N*4*-Diethyl-6-(4-methoxy-phen...)Show InChI InChI=1S/C17H20N6O/c1-4-23(5-2)16-14-15(21-17(18)22-16)19-10-13(20-14)11-6-8-12(24-3)9-7-11/h6-10H,4-5H2,1-3H3,(H2,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115156

(4-(3-Trifluoromethyl-3H-diazirin-3-yl)-benzoic aci...)Show SMILES Nc1nc(O)c2NC(COC(=O)c3ccc(cc3)C3(N=N3)C(F)(F)F)CNc2n1 |c:20| Show InChI InChI=1S/C16H14F3N7O3/c17-16(18,19)15(25-26-15)8-3-1-7(2-4-8)13(28)29-6-9-5-21-11-10(22-9)12(27)24-14(20)23-11/h1-4,9,22H,5-6H2,(H4,20,21,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115148
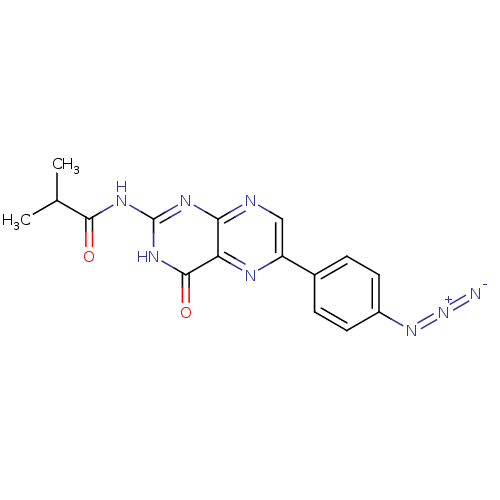
(CHEMBL101037 | N-[6-(4-Azido-phenyl)-4-oxo-3,4-dih...)Show SMILES CC(C)C(=O)Nc1nc2ncc(nc2c(=O)[nH]1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C16H14N8O2/c1-8(2)14(25)21-16-20-13-12(15(26)22-16)19-11(7-18-13)9-3-5-10(6-4-9)23-24-17/h3-8H,1-2H3,(H2,18,20,21,22,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115140

(6-(4-Methoxy-phenyl)-4-piperidin-1-yl-5,6,7,8-tetr...)Show InChI InChI=1S/C18H24N6O/c1-25-13-7-5-12(6-8-13)14-11-20-16-15(21-14)17(23-18(19)22-16)24-9-3-2-4-10-24/h5-8,14,21H,2-4,9-11H2,1H3,(H3,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115153

(6-(3,4-Dimethoxy-phenyl)-4-piperidin-1-yl-pteridin...)Show InChI InChI=1S/C19H22N6O2/c1-26-14-7-6-12(10-15(14)27-2)13-11-21-17-16(22-13)18(24-19(20)23-17)25-8-4-3-5-9-25/h6-7,10-11H,3-5,8-9H2,1-2H3,(H2,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115143
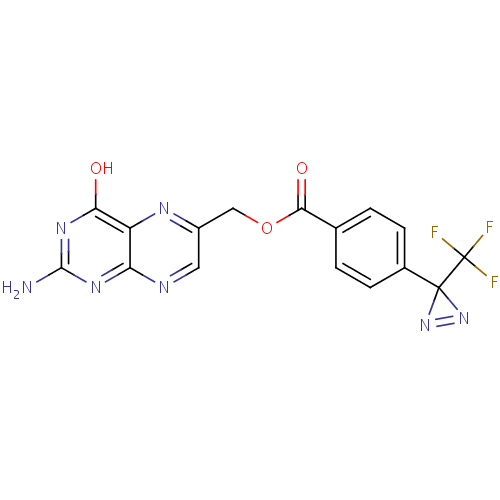
(4-(3-Trifluoromethyl-3H-diazirin-3-yl)-benzoic aci...)Show SMILES Nc1nc(O)c2nc(COC(=O)c3ccc(cc3)C3(N=N3)C(F)(F)F)cnc2n1 |c:20| Show InChI InChI=1S/C16H10F3N7O3/c17-16(18,19)15(25-26-15)8-3-1-7(2-4-8)13(28)29-6-9-5-21-11-10(22-9)12(27)24-14(20)23-11/h1-5H,6H2,(H3,20,21,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115147
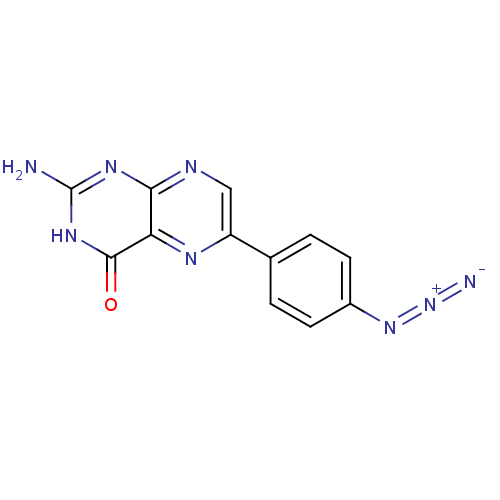
(2-Amino-6-(4-azido-phenyl)-3H-pteridin-4-one | CHE...)Show SMILES Nc1nc2ncc(nc2c(=O)[nH]1)-c1ccc(cc1)N=[N+]=[N-] Show InChI InChI=1S/C12H8N8O/c13-12-17-10-9(11(21)18-12)16-8(5-15-10)6-1-3-7(4-2-6)19-20-14/h1-5H,(H3,13,15,17,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115124
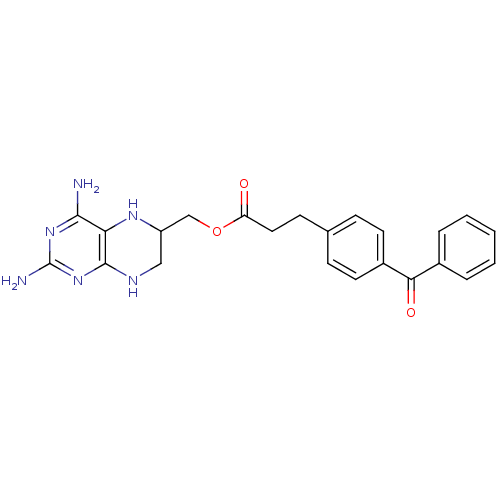
(3-(4-Benzoyl-phenyl)-propionic acid 2,4-diamino-5,...)Show SMILES Nc1nc(N)c2NC(COC(=O)CCc3ccc(cc3)C(=O)c3ccccc3)CNc2n1 Show InChI InChI=1S/C23H24N6O3/c24-21-19-22(29-23(25)28-21)26-12-17(27-19)13-32-18(30)11-8-14-6-9-16(10-7-14)20(31)15-4-2-1-3-5-15/h1-7,9-10,17,27H,8,11-13H2,(H5,24,25,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115167
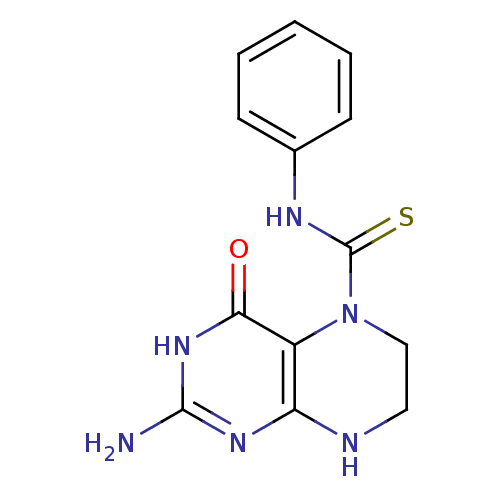
(2-Amino-4-oxo-4,6,7,8-tetrahydro-3H-pteridine-5-ca...)Show InChI InChI=1S/C13H14N6OS/c14-12-17-10-9(11(20)18-12)19(7-6-15-10)13(21)16-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,16,21)(H4,14,15,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081590

(6-(4-Methoxy-phenyl)-4-piperidin-1-yl-pteridin-2-y...)Show InChI InChI=1S/C18H20N6O/c1-25-13-7-5-12(6-8-13)14-11-20-16-15(21-14)17(23-18(19)22-16)24-9-3-2-4-10-24/h5-8,11H,2-4,9-10H2,1H3,(H2,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115169
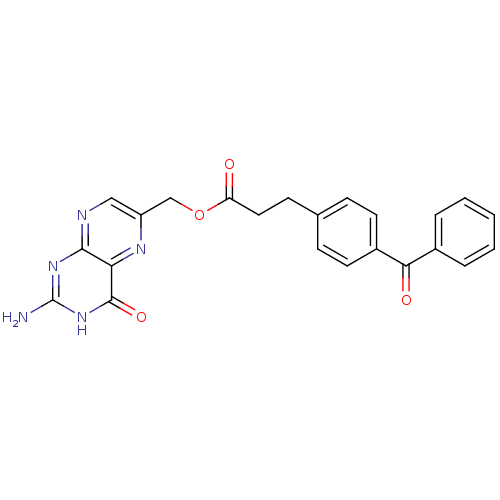
(3-(4-Benzoyl-phenyl)-propionic acid 2-amino-4-oxo-...)Show SMILES Nc1nc2ncc(COC(=O)CCc3ccc(cc3)C(=O)c3ccccc3)nc2c(=O)[nH]1 Show InChI InChI=1S/C23H19N5O4/c24-23-27-21-19(22(31)28-23)26-17(12-25-21)13-32-18(29)11-8-14-6-9-16(10-7-14)20(30)15-4-2-1-3-5-15/h1-7,9-10,12H,8,11,13H2,(H3,24,25,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115137
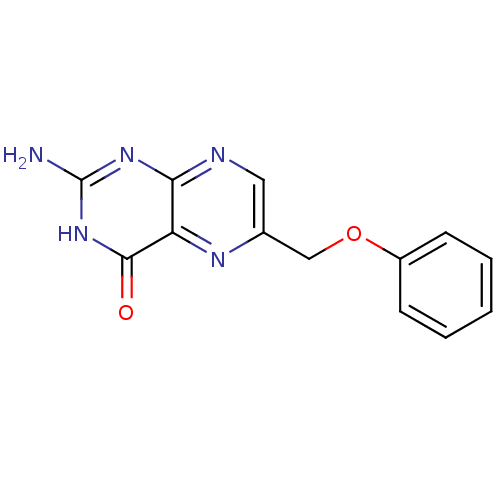
(2-Amino-6-phenoxymethyl-3H-pteridin-4-one | CHEMBL...)Show InChI InChI=1S/C13H11N5O2/c14-13-17-11-10(12(19)18-13)16-8(6-15-11)7-20-9-4-2-1-3-5-9/h1-6H,7H2,(H3,14,15,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50081580
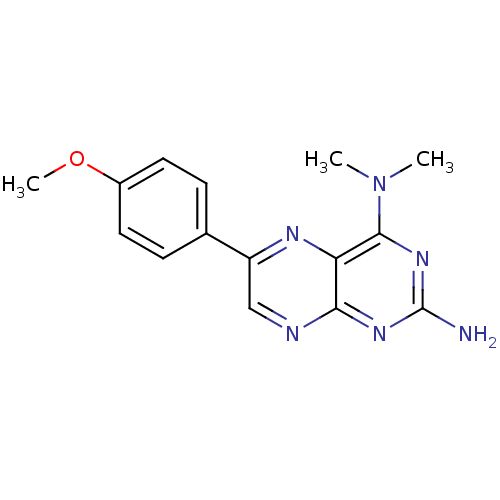
(6-(4-Methoxy-phenyl)-N*4*,N*4*-dimethyl-pteridine-...)Show InChI InChI=1S/C15H16N6O/c1-21(2)14-12-13(19-15(16)20-14)17-8-11(18-12)9-4-6-10(22-3)7-5-9/h4-8H,1-3H3,(H2,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115157
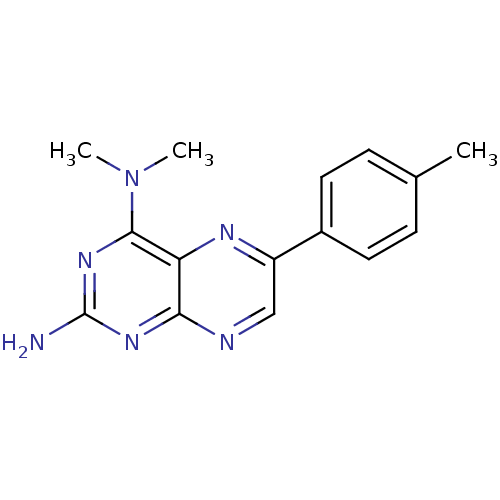
(CHEMBL101513 | N*4*,N*4*-Dimethyl-6-p-tolyl-pterid...)Show InChI InChI=1S/C15H16N6/c1-9-4-6-10(7-5-9)11-8-17-13-12(18-11)14(21(2)3)20-15(16)19-13/h4-8H,1-3H3,(H2,16,17,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50115173
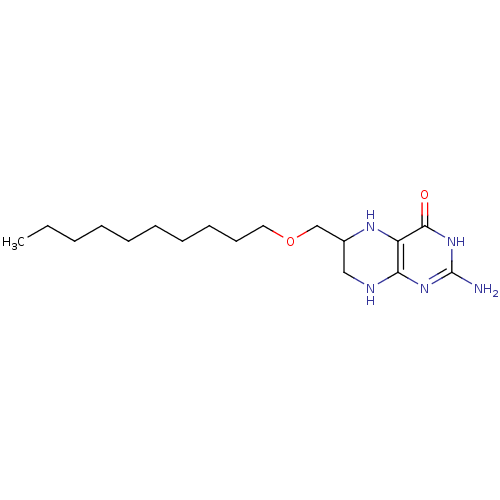
(2-Amino-6-decyloxymethyl-5,6,7,8-tetrahydro-3H-pte...)Show InChI InChI=1S/C17H31N5O2/c1-2-3-4-5-6-7-8-9-10-24-12-13-11-19-15-14(20-13)16(23)22-17(18)21-15/h13,20H,2-12H2,1H3,(H4,18,19,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (NOS-I) |
J Med Chem 45: 2923-41 (2002)
BindingDB Entry DOI: 10.7270/Q2571BBK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data