 Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50013431
Found 42 hits Enz. Inhib. hit(s) with all data for entry = 50013431 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50565800
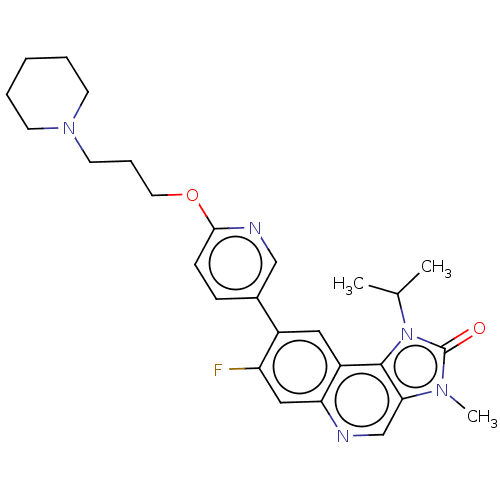
(Azd-1390 | Azd1390)Show SMILES CC(C)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN3CCCCC3)nc2)n(C)c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATM (unknown origin) assessed as ATM-dependent phosphorylation using GST-p53 ser15 as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | BDBM50565801
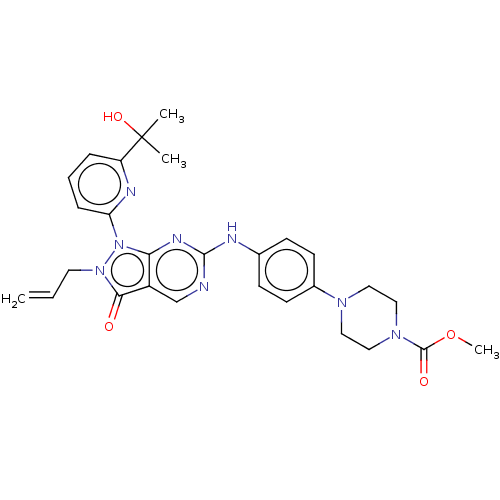
(CHEMBL4780868)Show SMILES COC(=O)N1CCN(CC1)c1ccc(Nc2ncc3c(n2)n(-c2cccc(n2)C(C)(C)O)n(CC=C)c3=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of kinase tracer178 from recombinant WEE1 (unknown origin) incubated for 1 hr by lanthascreen TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM50110178
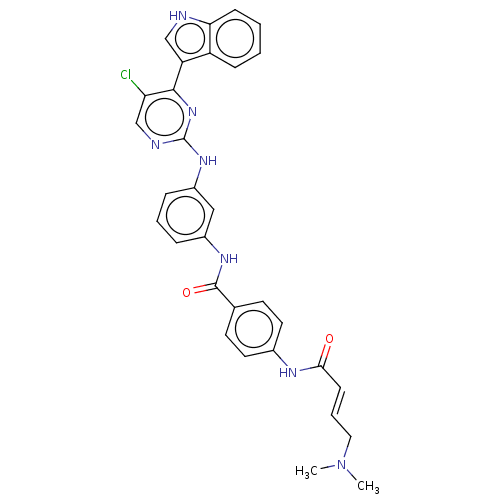
(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CDK7 incubated for 180 mins by LanthaScreen Eu Kinase binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | BDBM50240826
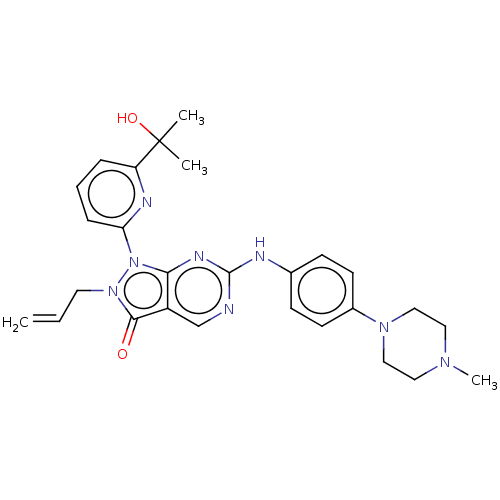
(AZD-1775 | MK-1775 | US11124518, Example AZD1775 |...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3c(n2)n(-c2cccc(n2)C(C)(C)O)n(CC=C)c3=O)cc1 Show InChI InChI=1S/C27H32N8O2/c1-5-13-34-25(36)21-18-28-26(29-19-9-11-20(12-10-19)33-16-14-32(4)15-17-33)31-24(21)35(34)23-8-6-7-22(30-23)27(2,3)37/h5-12,18,37H,1,13-17H2,2-4H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-fused human WEE1 (215 to 646 residues) expressed in baculovirus expression system using poly(Lys,Tyr) as substrate incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50565799
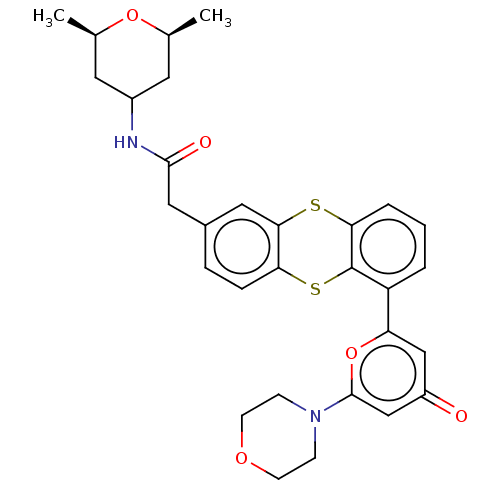
(CHEMBL4780892)Show SMILES C[C@H]1CC(C[C@@H](C)O1)NC(=O)Cc1ccc2Sc3c(Sc2c1)cccc3-c1cc(=O)cc(o1)N1CCOCC1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ATM (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
ATR-interacting protein/Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM268079

(2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...)Show SMILES C[C@@H]1COCCN1c1cc(-c2ccnn2C)c2ccnc(-c3ccn[nH]3)c2n1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 5-TAMRA-labelled Tracer A from human GST-fused/FLAG-tagged full length ATR/human N-terminal FLAG-tagged full length ATRIP expressed i... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50145038

(CHEMBL2143829)Show InChI InChI=1S/C17H15N7O2/c1-25-13-7-10-12(8-14(13)26-2)20-9-21-16(10)24-17(18)22-15(23-24)11-5-3-4-6-19-11/h3-9H,1-2H3,(H2,18,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length Flag-tagged ATM (unknown origin) using GST-p53(1 to 101 residues) as substrates incubated for 90 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50208517
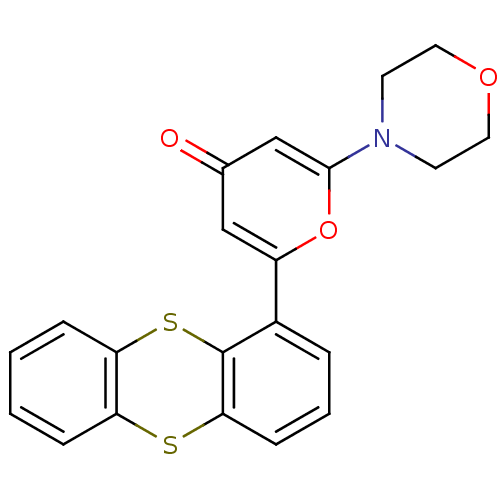
(2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one | C...)Show SMILES O=c1cc(oc(c1)-c1cccc2Sc3ccccc3Sc12)N1CCOCC1 Show InChI InChI=1S/C21H17NO3S2/c23-14-12-16(25-20(13-14)22-8-10-24-11-9-22)15-4-3-7-19-21(15)27-18-6-2-1-5-17(18)26-19/h1-7,12-13H,8-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HeLa nuclear extract derived ATM using glutathioneS-transferase-p53N66 as substrate preincubated for 10 mins followed by ATP addi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Wee1-like protein kinase
(Homo sapiens (Human)) | BDBM3096
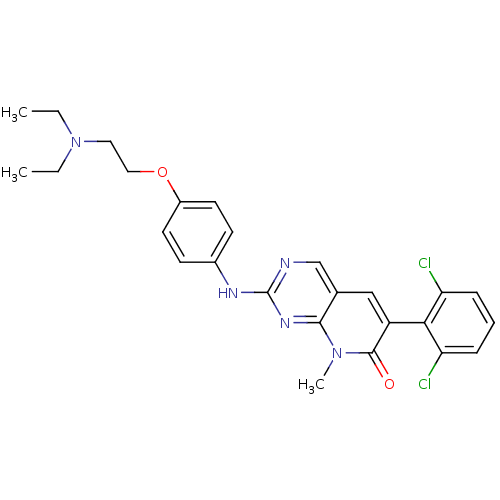
(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length WEE1 (unknown origin) assessed as reduction in cdc2/cyclin B phosphorylation using biotinylated histone H1 peptide (PKTPKKA... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50139171
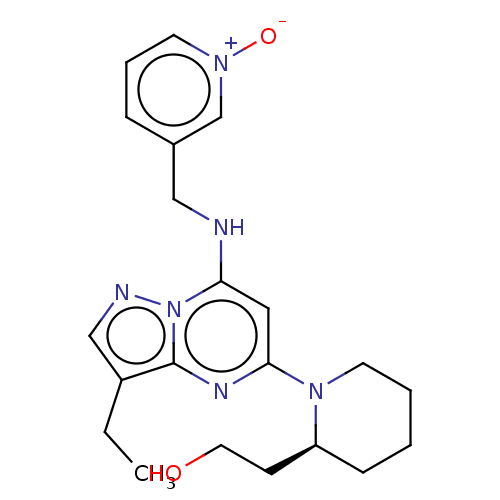
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CDK12 (696 to 1082 residues)/human cyclinK (1 to 267 residues) using pol2 CTD-peptide substrate and [gammaP]ATP by scintillation ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13/Cyclin-K
(Homo sapiens (Human)) | BDBM50528813
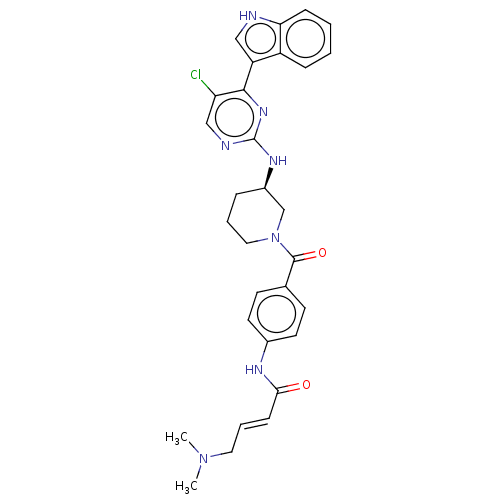
(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK13/human cyclin K expressed in baculovirus-infected insect cells using pol2 CTD-peptide substrate and [gammaP]ATP by scintillation c... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM413450

(7-methyl-2-((7-methyl-[1,2,4]triazolo[1,5-a]pyridi...)Show SMILES Cc1cc2ncnn2cc1Nc1ncc2n(C)c(=O)n(C3CCOCC3)c2n1 Show InChI InChI=1S/C18H20N8O2/c1-11-7-15-20-10-21-25(15)9-13(11)22-17-19-8-14-16(23-17)26(18(27)24(14)2)12-3-5-28-6-4-12/h7-10,12H,3-6H2,1-2H3,(H,19,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DNA-PK in human A549 cells assessed as reduction in radiation-induced autophosphorylation at S2056 residue preincubated for 1 hr follow... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of c-SRC (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EGFR (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FGFR1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MYT1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDGFR-beta (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM3096

(2-aminopyrido[2,3-d]pyrimidin-7(8H)-one deriv. | 6...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4c(Cl)cccc4Cl)c(=O)n(C)c3n2)cc1 |(-8.3,-10.68,;-6.82,-11.08,;-5.73,-9.99,;-4.24,-10.39,;-3.15,-9.3,;-6.82,-8.9,;-6.82,-7.36,;-8.15,-6.59,;-8.15,-5.05,;-6.82,-4.28,;-6.82,-2.74,;-8.15,-1.97,;-8.15,-.43,;-6.82,.34,;-6.82,1.88,;-5.48,2.65,;-4.15,1.88,;-2.82,2.65,;-1.48,1.88,;-.15,2.65,;-.15,4.19,;-1.48,4.96,;1.18,4.96,;2.52,4.19,;2.52,2.65,;1.18,1.88,;1.18,.34,;-1.48,.34,;-.15,-.43,;-2.82,-.43,;-2.82,-1.97,;-4.15,.34,;-5.48,-.43,;-9.48,-2.74,;-9.48,-4.28,)| Show InChI InChI=1S/C26H27Cl2N5O2/c1-4-33(5-2)13-14-35-19-11-9-18(10-12-19)30-26-29-16-17-15-20(25(34)32(3)24(17)31-26)23-21(27)7-6-8-22(23)28/h6-12,15-16H,4-5,13-14H2,1-3H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50528813

(CHEMBL4163879)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H](C1)Nc1ncc(Cl)c(n1)-c1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C30H32ClN7O2/c1-37(2)15-6-10-27(39)34-21-13-11-20(12-14-21)29(40)38-16-5-7-22(19-38)35-30-33-18-25(31)28(36-30)24-17-32-26-9-4-3-8-23(24)26/h3-4,6,8-14,17-18,22,32H,5,7,15-16,19H2,1-2H3,(H,34,39)(H,33,35,36)/b10-6+/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CDK12/human cyclin K expressed in baculovirus-infected insect cells using pol2 CTD-peptide substrate and [gammaP]ATP by scintilla... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 13
(Homo sapiens (Human)) | BDBM50110178

(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK13 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His-tagged human RD52 assessed as reduction in RD52 binding to Cy3-dT30-Cy5 ssDNA incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K alpha (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K delta (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 12
(Homo sapiens) | BDBM50110178

(CHEMBL3603847 | US10787436, Compound I-23)Show SMILES CN(C)C\C=C\C(=O)Nc1ccc(cc1)C(=O)Nc1cccc(Nc2ncc(Cl)c(n2)-c2c[nH]c3ccccc23)c1 Show InChI InChI=1S/C31H28ClN7O2/c1-39(2)16-6-11-28(40)35-21-14-12-20(13-15-21)30(41)36-22-7-5-8-23(17-22)37-31-34-19-26(32)29(38-31)25-18-33-27-10-4-3-9-24(25)27/h3-15,17-19,33H,16H2,1-2H3,(H,35,40)(H,36,41)(H,34,37,38)/b11-6+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 864 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK12 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K beta (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50436204

(CHEMBL109037)Show InChI InChI=1S/C9H11NO5/c10-5(9(14)15)1-4-2-7(12)8(13)3-6(4)11/h2-3,5,11-13H,1,10H2,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565810
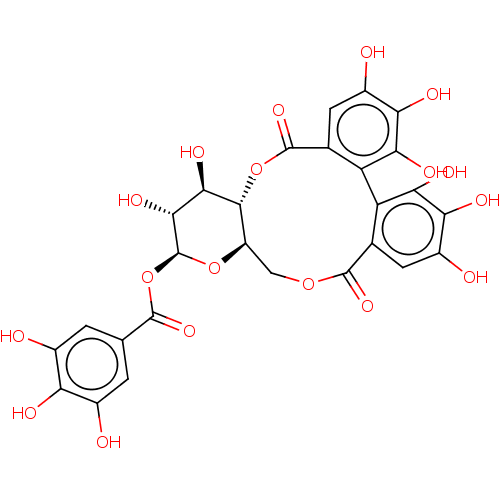
(Strictinin)Show SMILES [H][C@@]12COC(=O)c3cc(O)c(O)c(O)c3-c3c(O)c(O)c(O)cc3C(=O)O[C@@]1([H])[C@H](O)[C@@H](O)[C@H](OC(=O)c1cc(O)c(O)c(O)c1)O2 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His-tagged human RD52 assessed as reduction in RD52 binding to Cy3-dT30-Cy5 ssDNA incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50187665
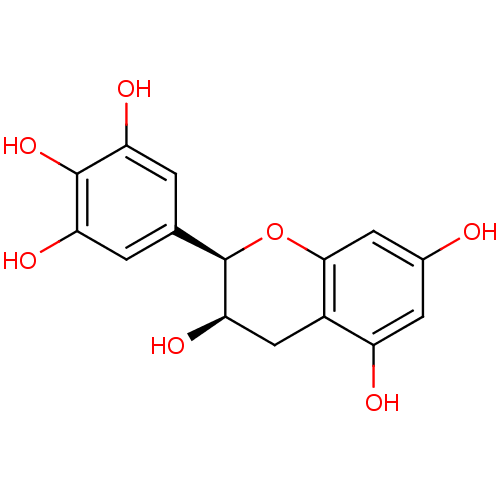
((-)-epigallocatechin | CHEMBL47386)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 6His-tagged human RD52 assessed as reduction in RD52 binding to Cy3-dT30-Cy5 ssDNA incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50565798
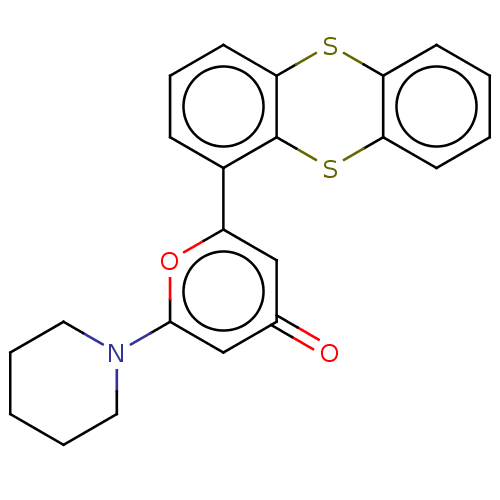
(CHEMBL4789596)Show SMILES O=c1cc(oc(c1)-c1cccc2Sc3ccccc3Sc12)N1CCCCC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HeLa nuclear extract derived ATM using glutathioneS-transferase-p53N66 as substrate preincubated for 10 mins followed by ATP addi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565806
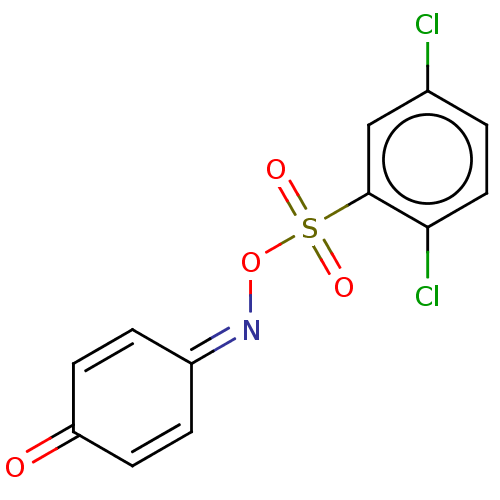
(CHEMBL4795092)Show SMILES Clc1ccc(Cl)c(c1)S(=O)(=O)[#8]\[#7]=[#6]-1/[#6]=[#6]-[#6](=O)-[#6]=[#6]-1 |c:15,19| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565807
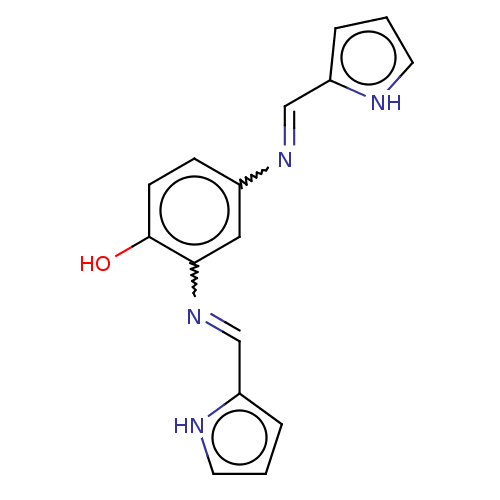
(CHEMBL4785174) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565808
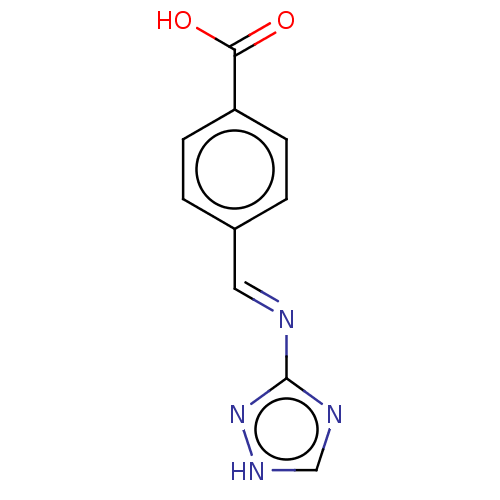
(CHEMBL4785369) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM23223
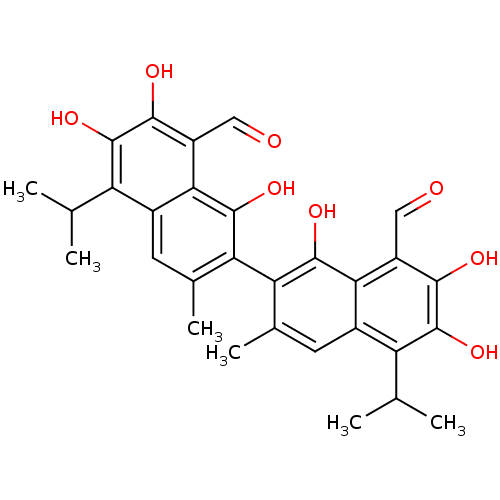
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565809
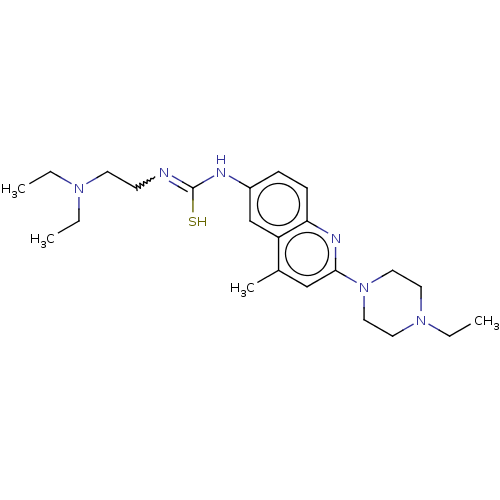
(CHEMBL1718911)Show SMILES CCN(CC)CCN=C(S)Nc1ccc2nc(cc(C)c2c1)N1CCN(CC)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human RAD52 assessed as reduction in DNA annealing using tailed ds-DNA 337-F/1337-BHQ1 by fluorescence-quenching assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565805
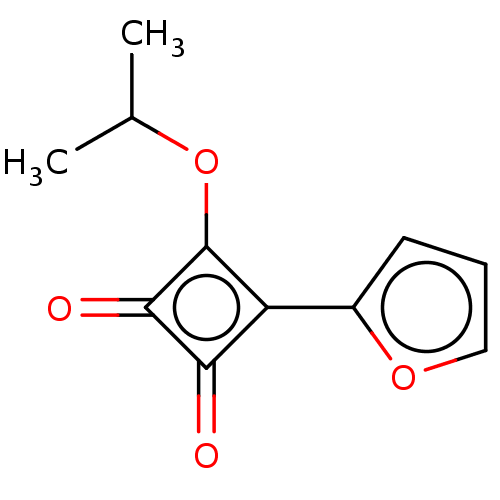
(CHEMBL4785153) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565804
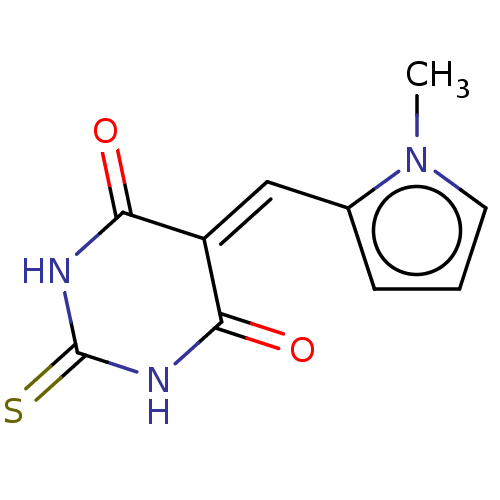
(CHEMBL4796552)Show SMILES [#6]-n1cccc1\[#6]=[#6]-1\[#6](=O)-[#7]-[#6](=S)-[#7]-[#6]-1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565803
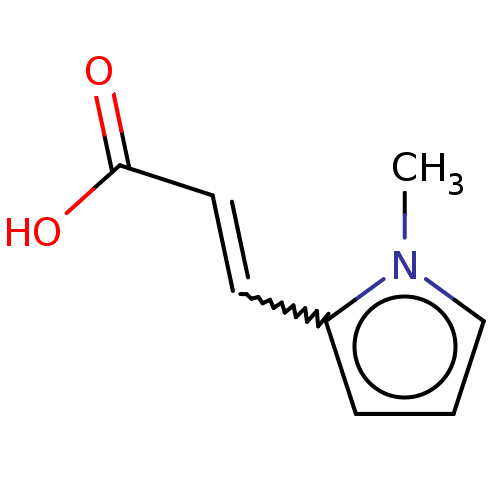
(CHEMBL4777728) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM60996

(5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...)Show SMILES [#8]-[#6](=O)-[#6]-1=[#6]\[#6](-[#6]=[#6]-[#6]-1=O)=[#6](\c1ccc(-[#8])c(c1)-[#6](-[#8])=O)-c1ccc(-[#8])c(c1)-[#6](-[#8])=O |c:6,t:3| Show InChI InChI=1S/C22H14O9/c23-16-4-1-10(7-13(16)20(26)27)19(11-2-5-17(24)14(8-11)21(28)29)12-3-6-18(25)15(9-12)22(30)31/h1-9,23-24H,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD52 homolog
(Homo sapiens) | BDBM50565802
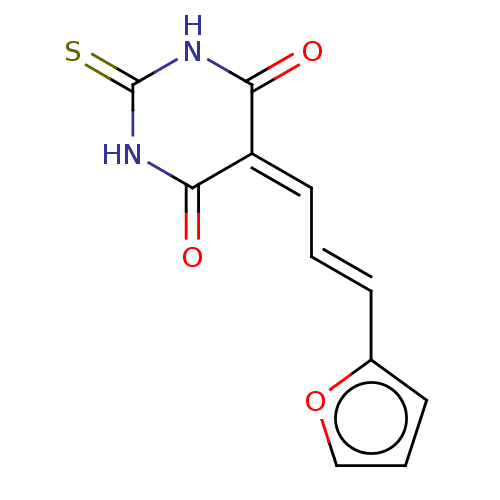
(CHEMBL4165444 | US11584714, Compound 29)Show SMILES O=[#6]1-[#7]-[#6](=S)-[#7]-[#6](=O)\[#6]-1=[#6]\[#6]=[#6]\c1ccco1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of FAM-conjugated ssDNA binding to His-tagged wild type RAD52 (unknown origin) expressed in Rosetta2(DE3)/pLysS cells measured after 30 mi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565800

(Azd-1390 | Azd1390)Show SMILES CC(C)n1c2c(cnc3cc(F)c(cc23)-c2ccc(OCCCN3CCCCC3)nc2)n(C)c1=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
DNA repair protein RAD51 homolog 1
(Homo sapiens (Human)) | BDBM48804
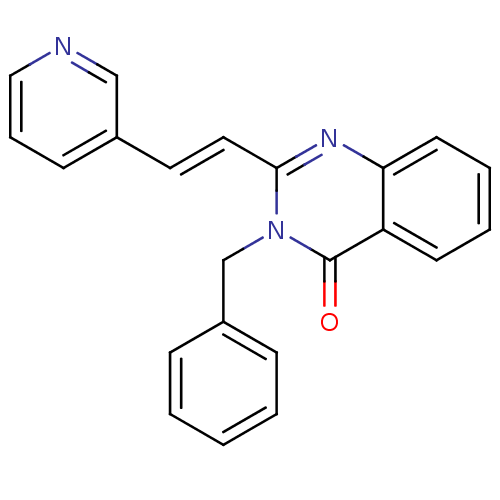
(3-(phenylmethyl)-2-[(E)-2-(3-pyridinyl)ethenyl]-4-...)Show InChI InChI=1S/C22H17N3O/c26-22-19-10-4-5-11-20(19)24-21(13-12-17-9-6-14-23-15-17)25(22)16-18-7-2-1-3-8-18/h1-15H,16H2/b13-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human RAD51 using 90-mer ssDNA as substrate measured after 30 mins by D-loop assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM245474

(US9428503, 1)Show SMILES CN(C)CCCOc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2c1 Show InChI InChI=1S/C26H31N5O3/c1-29(2)11-4-12-34-24-8-6-19(16-28-24)18-5-7-22-21(15-18)25-23(17-27-22)30(3)26(32)31(25)20-9-13-33-14-10-20/h5-8,15-17,20H,4,9-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00766
BindingDB Entry DOI: 10.7270/Q2GX4G9M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data