 Found 91 hits Enz. Inhib. hit(s) with all data for entry = 50016242
Found 91 hits Enz. Inhib. hit(s) with all data for entry = 50016242 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
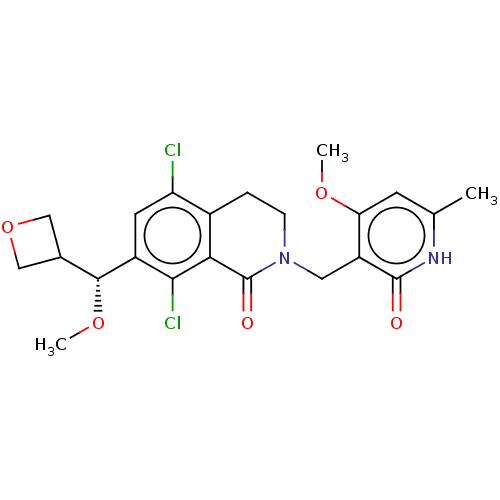
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50272462
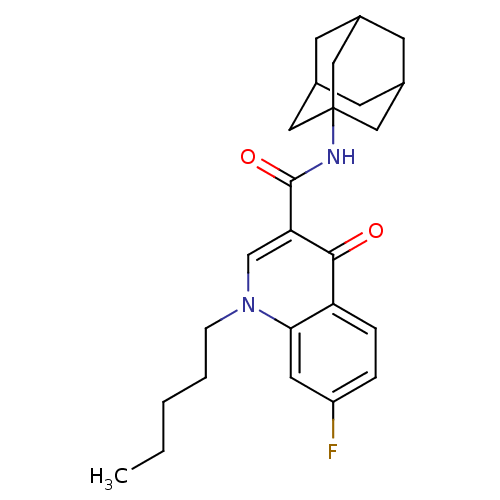
(CHEMBL525528 | N-(Adamant-1-yl)-7-fluoro-4-oxo-1-p...)Show SMILES CCCCCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)c2ccc(F)cc12 |TLB:10:11:14:18.16.17,THB:16:15:12:18.17.19,16:17:14.15.20:12,19:17:14:20.11.12,19:11:14:18.16.17| Show InChI InChI=1S/C25H31FN2O2/c1-2-3-4-7-28-15-21(23(29)20-6-5-19(26)11-22(20)28)24(30)27-25-12-16-8-17(13-25)10-18(9-16)14-25/h5-6,11,15-18H,2-4,7-10,12-14H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50156024
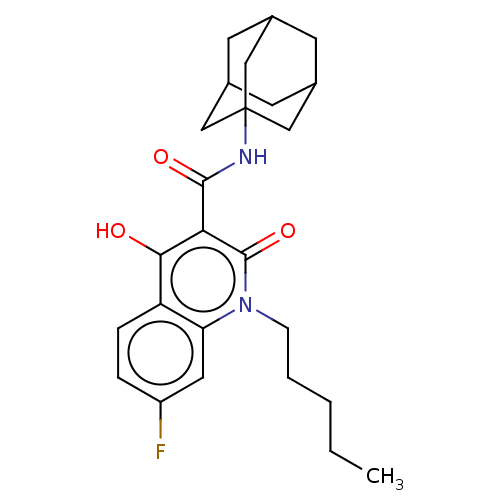
(CHEMBL3780852)Show SMILES CCCCCn1c2cc(F)ccc2c(O)c(C(=O)NC23CC4CC(CC(C4)C2)C3)c1=O |TLB:26:25:28:22.21.20,26:21:28:25.27.24,THB:24:25:22.23.28:20,24:23:20:25.27.26| Show InChI InChI=1S/C31H37NO11S/c1-14(33)39-24-25(40-15(2)34)27(41-16(3)35)30(43-26(24)29(36)38-6)44-21-10-8-18-19-13-17-7-9-20(37-5)23-22(17)31(18,28(21)42-23)11-12-32(19)4/h7-10,18-19,21,24-28,30H,11-13H2,1-6H3/t18-,19+,21?,24?,25?,26?,27?,28-,30?,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50193709
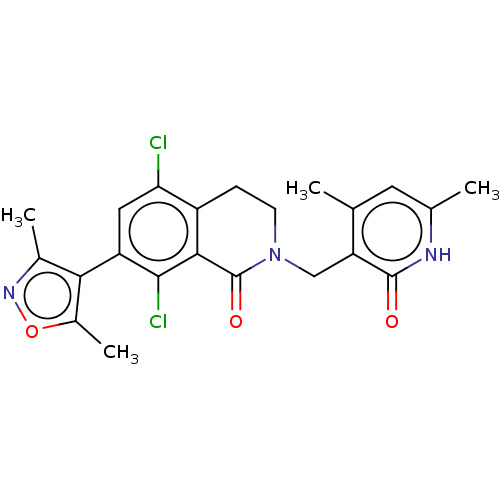
(CHEMBL3911017)Show SMILES Cc1noc(C)c1-c1cc(Cl)c2CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c2c1Cl |(59.06,-26.01,;58.74,-27.52,;59.77,-28.66,;59,-30,;57.5,-29.68,;56.35,-30.71,;57.33,-28.15,;55.99,-27.38,;54.66,-28.14,;53.34,-27.37,;52.01,-28.14,;53.35,-25.85,;52.02,-25.09,;52.02,-23.55,;53.35,-22.77,;53.35,-21.23,;52.02,-20.46,;52.02,-18.92,;53.36,-18.16,;50.7,-18.15,;49.36,-18.92,;48.03,-18.14,;49.35,-20.46,;50.69,-21.24,;50.69,-22.78,;54.68,-23.55,;56.01,-22.79,;54.68,-25.09,;55.99,-25.86,;57.33,-25.09,)| Show InChI InChI=1S/C22H21Cl2N3O3/c1-10-7-11(2)25-21(28)16(10)9-27-6-5-14-17(23)8-15(20(24)19(14)22(27)29)18-12(3)26-30-13(18)4/h7-8H,5-6,9H2,1-4H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50206092
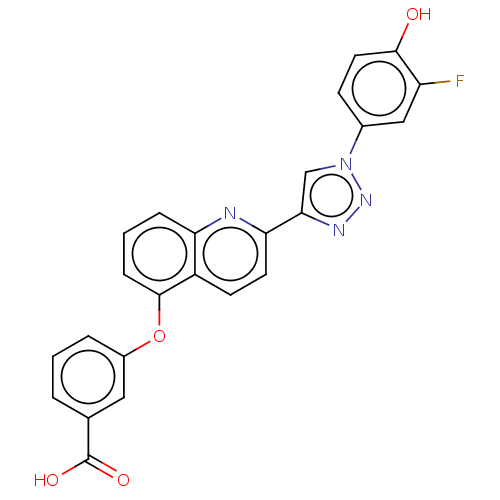
(CHEMBL3917143 | US10336721, Example 32 | US1096819...)Show SMILES OC(=O)c1cccc(Oc2cccc3nc(ccc23)-c2cn(nn2)-c2ccc(O)c(F)c2)c1 Show InChI InChI=1S/C24H15FN4O4/c25-18-12-15(7-10-22(18)30)29-13-21(27-28-29)20-9-8-17-19(26-20)5-2-6-23(17)33-16-4-1-3-14(11-16)24(31)32/h1-13,30H,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50593504
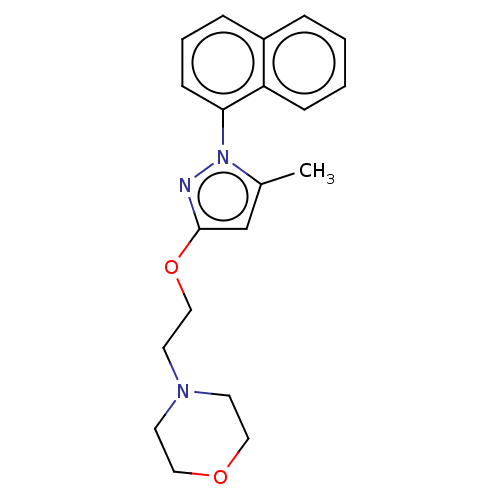
(CHEMBL5187095) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50593505
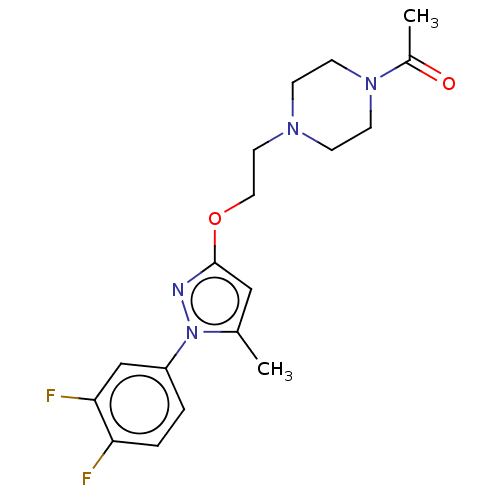
(CHEMBL5183349)Show SMILES CC(=O)N1CCN(CCOc2cc(C)n(n2)-c2ccc(F)c(F)c2)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50206062
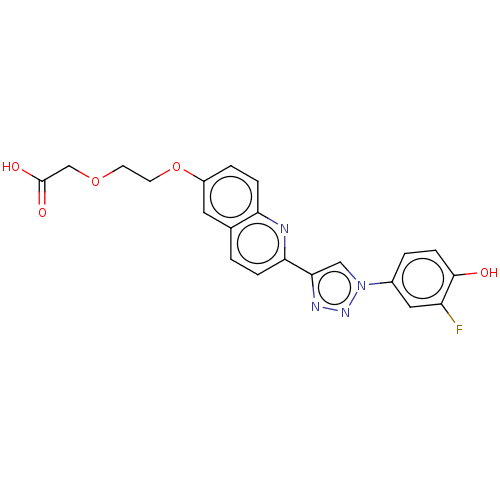
(CHEMBL3890541 | US10968198, Example 44)Show SMILES OC(=O)COCCOc1ccc2nc(ccc2c1)-c1cn(nn1)-c1ccc(O)c(F)c1 Show InChI InChI=1S/C21H17FN4O5/c22-16-10-14(2-6-20(16)27)26-11-19(24-25-26)18-4-1-13-9-15(3-5-17(13)23-18)31-8-7-30-12-21(28)29/h1-6,9-11,27H,7-8,12H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50156028
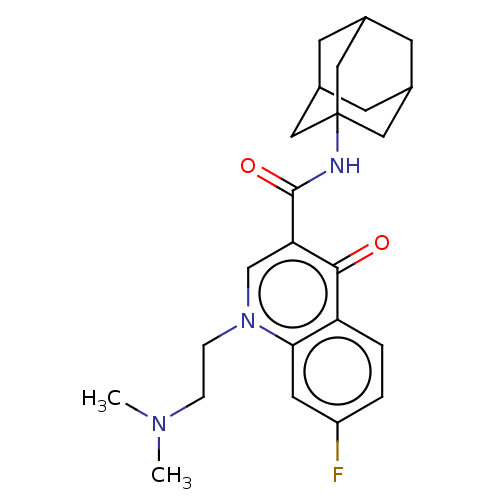
(CHEMBL3781544)Show SMILES CN(C)CCn1cc(C(=O)NC23CC4CC(CC(C4)C2)C3)c(=O)c2ccc(F)cc12 |TLB:18:17:20:14.13.12,18:13:20:17.19.16,THB:16:17:14.15.20:12,16:15:12:17.19.18| Show InChI InChI=1S/C33H39NO12S/c1-15(35)40-14-24-28(42-17(3)37)29(43-18(4)38)30(44-19(5)39)32(45-24)47-25-10-8-21-22-13-20-7-9-23(41-16(2)36)27-26(20)33(21,31(25)46-27)11-12-34(22)6/h7-10,21-22,24-25,28-32H,11-14H2,1-6H3/t21-,22+,24?,25?,28?,29?,30?,31-,32?,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50206061
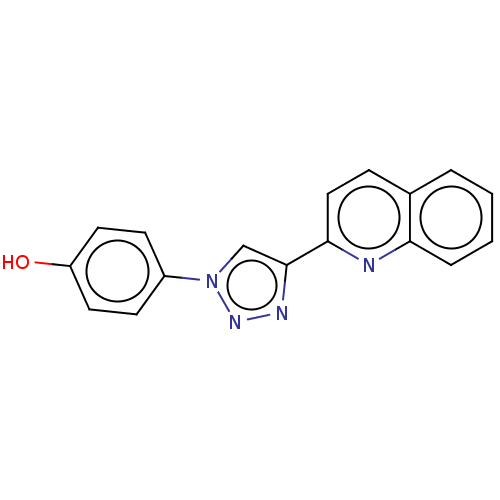
(CHEMBL3953102 | US10336721, Example 1 | US10968198...)Show InChI InChI=1S/C17H12N4O/c22-14-8-6-13(7-9-14)21-11-17(19-20-21)16-10-5-12-3-1-2-4-15(12)18-16/h1-11,22H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50593499
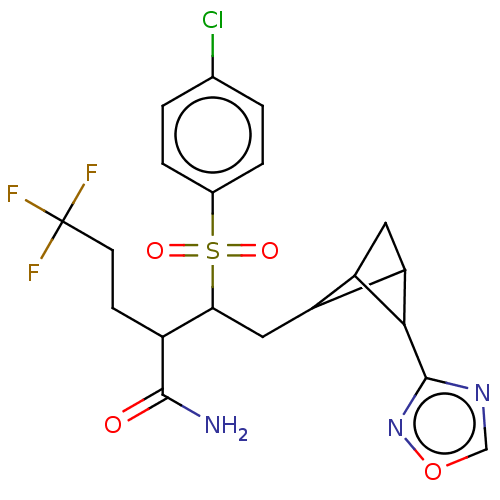
(CHEMBL5202466)Show SMILES NC(=O)C(CCC(F)(F)F)C(CC1C2CC1C2c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 |(3.51,-3.85,;2.17,-3.08,;.84,-3.85,;2.17,-1.54,;3.51,-.77,;4.84,-1.54,;6.18,-.77,;7.51,-1.54,;6.95,.57,;5.41,.57,;.84,-.77,;-.49,-1.54,;-1.83,-.77,;-3.34,-1.17,;-2.24,-.41,;-2.22,.72,;-3.74,.31,;-5.07,1.08,;-6.48,.46,;-7.51,1.6,;-6.74,2.94,;-5.23,2.62,;.84,.77,;.07,2.11,;-.7,.77,;2.17,1.54,;2.18,3.08,;3.51,3.85,;4.84,3.08,;6.17,3.85,;4.84,1.55,;3.51,.77,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.178 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50593498
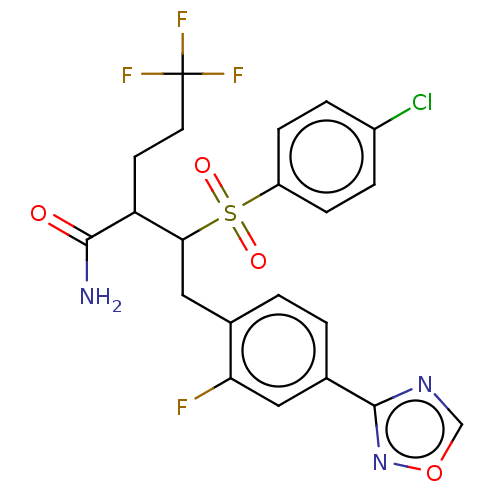
(CHEMBL5198066)Show SMILES NC(=O)C(CCC(F)(F)F)C(Cc1ccc(cc1F)-c1ncon1)S(=O)(=O)c1ccc(Cl)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428976
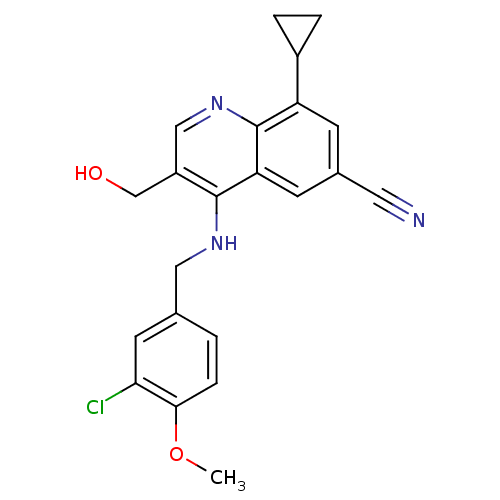
(CHEMBL2333219)Show SMILES COc1ccc(CNc2c(CO)cnc3c(cc(cc23)C#N)C2CC2)cc1Cl Show InChI InChI=1S/C22H20ClN3O2/c1-28-20-5-2-13(8-19(20)23)10-25-21-16(12-27)11-26-22-17(15-3-4-15)6-14(9-24)7-18(21)22/h2,5-8,11,15,27H,3-4,10,12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50593497

(CHEMBL5180183)Show SMILES O=C(NC1=CC=CC1)NC12CC3CC(CC(C3)C1)C2 |c:5,t:3,TLB:8:9:12.11.16:14,THB:10:11:14:18.9.17,10:9:12.11.16:14,17:9:12:16.15.14,17:15:12:18.10.9,8:9:12:16.15.14| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383292
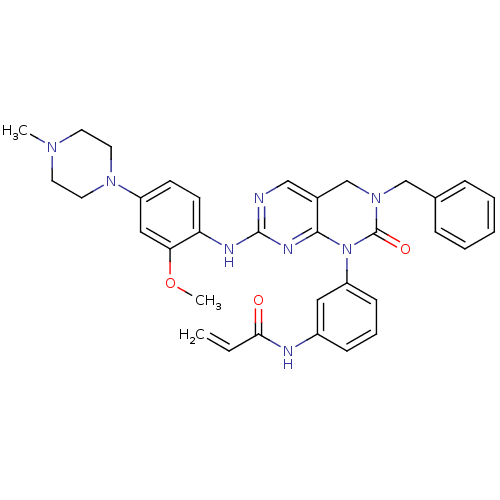
(CHEMBL2029438)Show SMILES COc1cc(ccc1Nc1ncc2CN(Cc3ccccc3)C(=O)N(c3cccc(NC(=O)C=C)c3)c2n1)N1CCN(C)CC1 Show InChI InChI=1S/C34H36N8O3/c1-4-31(43)36-26-11-8-12-28(19-26)42-32-25(23-41(34(42)44)22-24-9-6-5-7-10-24)21-35-33(38-32)37-29-14-13-27(20-30(29)45-3)40-17-15-39(2)16-18-40/h4-14,19-21H,1,15-18,22-23H2,2-3H3,(H,36,43)(H,35,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20557
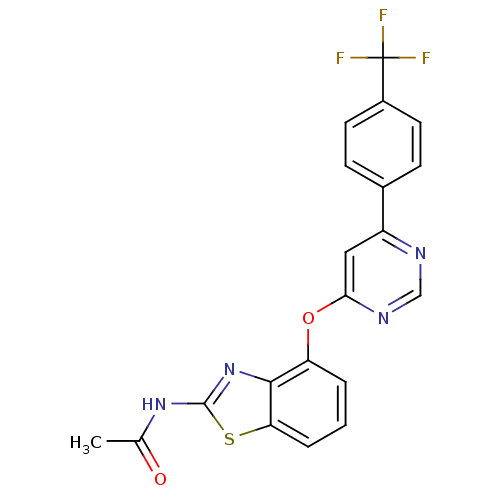
(AMG 517 | CHEMBL229430 | JMC503515 Compound 23 | N...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)-c3ccc(cc3)C(F)(F)F)cccc2s1 Show InChI InChI=1S/C20H13F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-10H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50593495

(CHEMBL5196156)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)C2NC1=NC2C=CC=C(C2=N1)c1ccc(F)c(F)c1F |r,c:23,25,28,t:20| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50108504
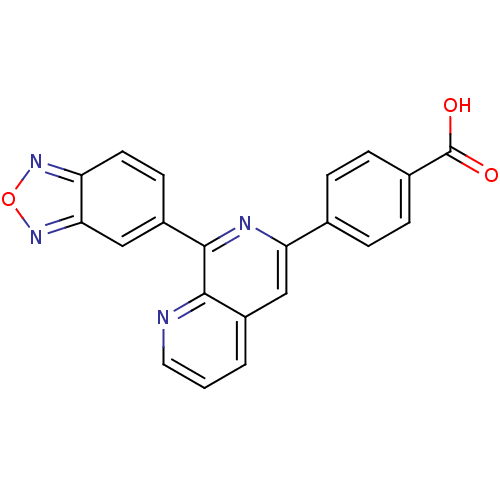
(4-(8-(benzo[c][1,2,5]oxadiazol-5-yl)-1,7-naphthyri...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1ccc2nonc2c1 Show InChI InChI=1S/C21H12N4O3/c26-21(27)13-5-3-12(4-6-13)17-10-14-2-1-9-22-19(14)20(23-17)15-7-8-16-18(11-15)25-28-24-16/h1-11H,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM16995
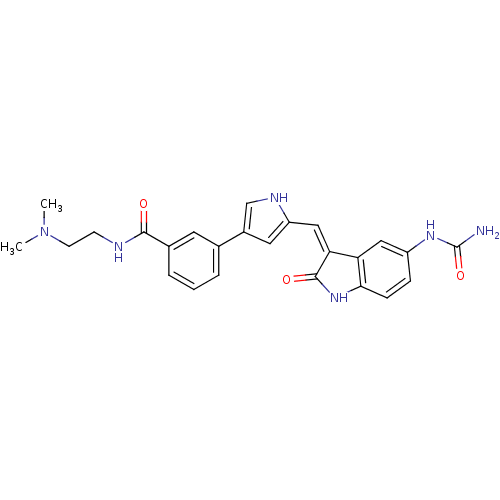
(3-(5-{[(3Z)-5-(carbamoylamino)-2-oxo-2,3-dihydro-1...)Show SMILES CN(C)CCNC(=O)c1cccc(c1)-c1c[nH]c(\C=C2/C(=O)Nc3ccc(NC(N)=O)cc23)c1 Show InChI InChI=1S/C25H26N6O3/c1-31(2)9-8-27-23(32)16-5-3-4-15(10-16)17-11-19(28-14-17)13-21-20-12-18(29-25(26)34)6-7-22(20)30-24(21)33/h3-7,10-14,28H,8-9H2,1-2H3,(H,27,32)(H,30,33)(H3,26,29,34)/b21-13- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50593495

(CHEMBL5196156)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)C2NC1=NC2C=CC=C(C2=N1)c1ccc(F)c(F)c1F |r,c:23,25,28,t:20| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM17009
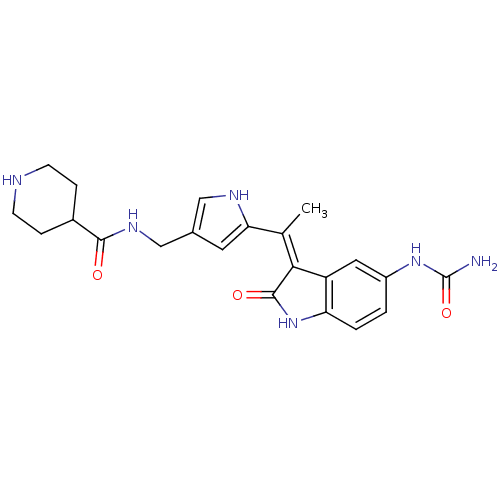
(Indolinone based compound, 17 | N-[(5-{1-[(3Z)-5-(...)Show SMILES C\C(=C1\C(=O)Nc2ccc(NC(N)=O)cc12)c1cc(CNC(=O)C2CCNCC2)c[nH]1 Show InChI InChI=1S/C22H26N6O3/c1-12(19-16-9-15(27-22(23)31)2-3-17(16)28-21(19)30)18-8-13(10-25-18)11-26-20(29)14-4-6-24-7-5-14/h2-3,8-10,14,24-25H,4-7,11H2,1H3,(H,26,29)(H,28,30)(H3,23,27,31)/b19-12- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1I
(Homo sapiens (Human)) | BDBM50200893
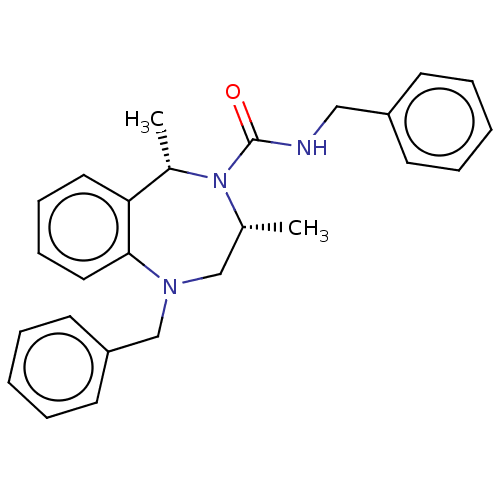
(CHEMBL3913893)Show SMILES C[C@@H]1CN(Cc2ccccc2)c2ccccc2[C@H](C)N1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C26H29N3O/c1-20-18-28(19-23-13-7-4-8-14-23)25-16-10-9-15-24(25)21(2)29(20)26(30)27-17-22-11-5-3-6-12-22/h3-16,20-21H,17-19H2,1-2H3,(H,27,30)/t20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50593491
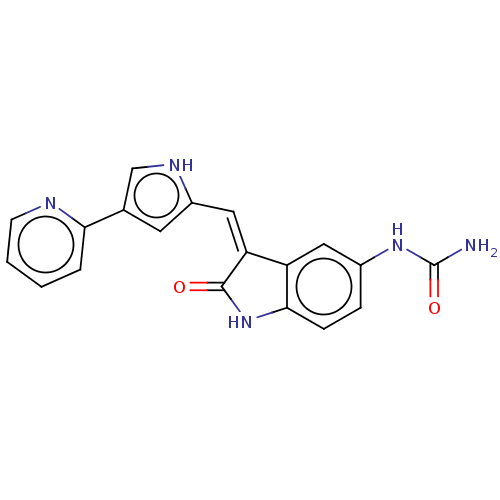
(CHEMBL5177707)Show SMILES NC(=O)Nc1ccc2NC(=O)\C(=C/c3cc(c[nH]3)-c3ccccn3)c2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50593494
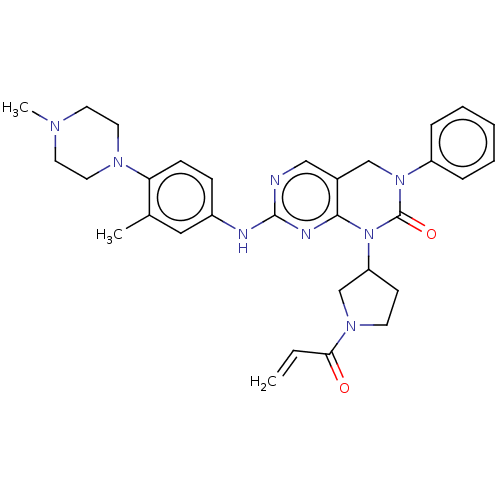
(CHEMBL5188568)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc3CN(C(=O)N(C4CCN(C4)C(=O)C=C)c3n2)c2ccccc2)cc1C | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50015448
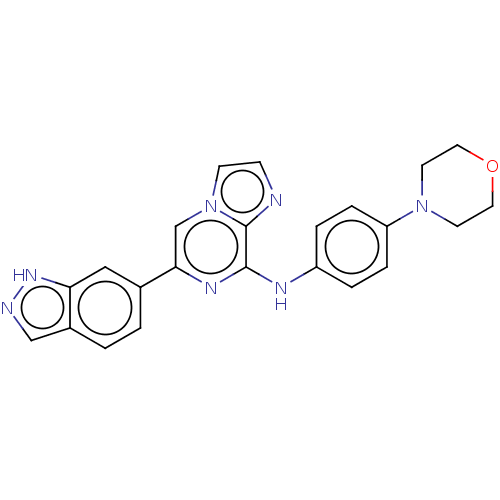
(CHEMBL3265032)Show SMILES C1CN(CCO1)c1ccc(Nc2nc(cn3ccnc23)-c2ccc3cn[nH]c3c2)cc1 Show InChI InChI=1S/C23H21N7O/c1-2-17-14-25-28-20(17)13-16(1)21-15-30-8-7-24-23(30)22(27-21)26-18-3-5-19(6-4-18)29-9-11-31-12-10-29/h1-8,13-15H,9-12H2,(H,25,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Presenilin-1
(Homo sapiens (Human)) | BDBM50593496
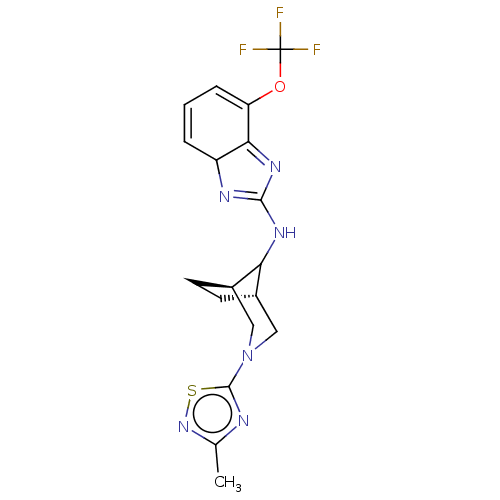
(CHEMBL5196077)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)C2NC1=NC2C=CC=C(OC(F)(F)F)C2=N1 |r,c:23,33,t:20,25| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50593489
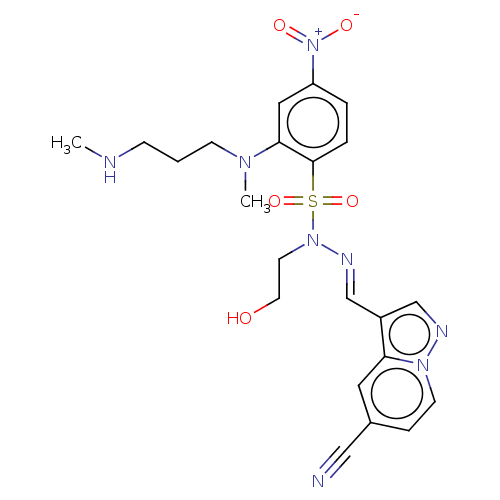
(CHEMBL5180410)Show SMILES CNCCCN(C)c1cc(ccc1S(=O)(=O)N(CCO)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM16981
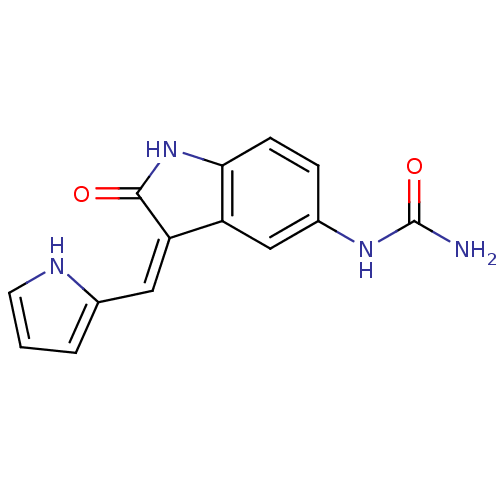
(BX-201 | [(3Z)-2-oxo-3-(1H-pyrrol-2-ylmethylidene)...)Show InChI InChI=1S/C14H12N4O2/c15-14(20)17-9-3-4-12-10(7-9)11(13(19)18-12)6-8-2-1-5-16-8/h1-7,16H,(H,18,19)(H3,15,17,20)/b11-6- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50593496

(CHEMBL5196077)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1nc(C)ns1)C2NC1=NC2C=CC=C(OC(F)(F)F)C2=N1 |r,c:23,33,t:20,25| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20561
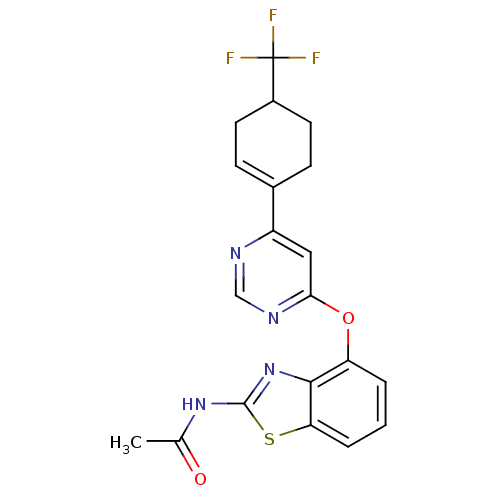
(N-[4-({6-[4-(trifluoromethyl)cyclohex-1-en-1-yl]py...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)C3=CCC(CC3)C(F)(F)F)cccc2s1 |t:16| Show InChI InChI=1S/C20H17F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-5,9-10,13H,6-8H2,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
O43924/P16499/P18545/P35913/P51160/Q13956
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50593492
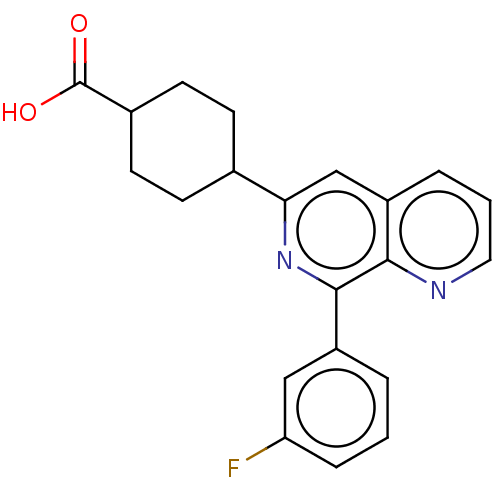
(CHEMBL4303204)Show SMILES OC(=O)C1CCC(CC1)c1cc2cccnc2c(n1)-c1cccc(F)c1 |(-6.4,5.84,;-5.06,6.61,;-5.06,8.15,;-3.73,5.84,;-3.73,4.3,;-2.4,3.53,;-1.06,4.3,;-1.06,5.84,;-2.4,6.61,;.27,3.53,;1.6,4.3,;2.94,3.53,;4.27,4.3,;5.6,3.52,;5.6,1.98,;4.27,1.22,;2.94,1.99,;1.6,1.22,;.27,1.99,;1.6,-.32,;.27,-1.09,;.26,-2.63,;1.6,-3.4,;2.93,-2.63,;4.26,-3.4,;2.93,-1.09,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50593508
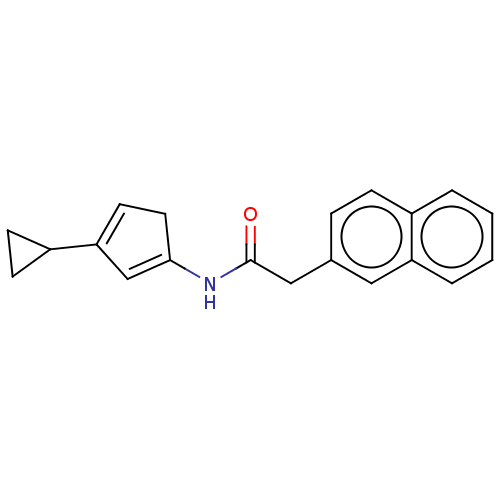
(CHEMBL5178051)Show SMILES O=C(Cc1ccc2ccccc2c1)NC1=CC(=CC1)C1CC1 |c:18,t:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50593509
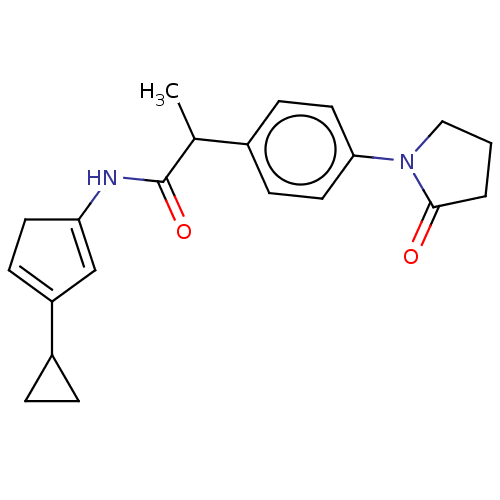
(CHEMBL5177155)Show SMILES CC(C(=O)NC1=CC(=CC1)C1CC1)c1ccc(cc1)N1CCCC1=O |c:7,t:5| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50133388

(CHEMBL3632824)Show SMILES NC(=O)c1cc(F)ccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C24H18FN3O4S/c25-17-6-7-18(19(12-17)22(26)29)15-3-1-14(2-4-15)13-33-24-28-27-23(32-24)16-5-8-20-21(11-16)31-10-9-30-20/h1-8,11-12H,9-10,13H2,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50461473

(CHEMBL4225824)Show SMILES CCCCCc1ccc(cc1)-c1cc(CCC(O)=O)c2c(c1)nc(N)n(CC1CCCO1)c2=O Show InChI InChI=1S/C27H33N3O4/c1-2-3-4-6-18-8-10-19(11-9-18)21-15-20(12-13-24(31)32)25-23(16-21)29-27(28)30(26(25)33)17-22-7-5-14-34-22/h8-11,15-16,22H,2-7,12-14,17H2,1H3,(H2,28,29)(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1I
(Homo sapiens (Human)) | BDBM50200854
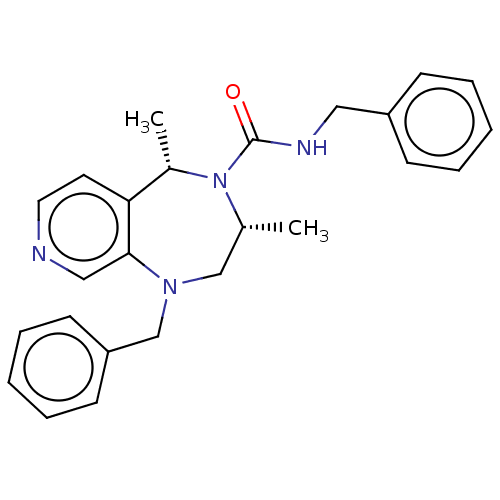
(CHEMBL3895005)Show SMILES C[C@@H]1CN(Cc2ccccc2)c2cnccc2[C@H](C)N1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C25H28N4O/c1-19-17-28(18-22-11-7-4-8-12-22)24-16-26-14-13-23(24)20(2)29(19)25(30)27-15-21-9-5-3-6-10-21/h3-14,16,19-20H,15,17-18H2,1-2H3,(H,27,30)/t19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50427326
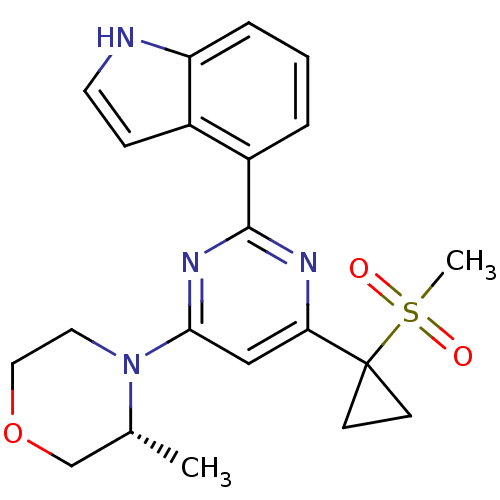
(CHEMBL2325697)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1cccc2[nH]ccc12)C1(CC1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H24N4O3S/c1-14-13-28-11-10-25(14)19-12-18(21(7-8-21)29(2,26)27)23-20(24-19)16-4-3-5-17-15(16)6-9-22-17/h3-6,9,12,14,22H,7-8,10-11,13H2,1-2H3/t14-/m1/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM50593500
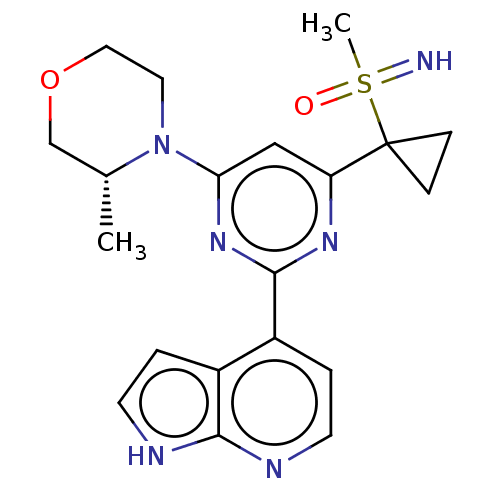
(CHEMBL5184230)Show SMILES C[C@@H]1COCCN1c1cc(nc(n1)-c1ccnc2[nH]ccc12)C1(CC1)S(C)(=N)=O |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50593488
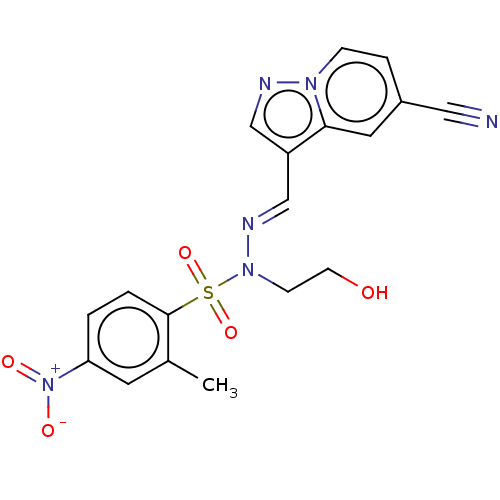
(CHEMBL5190501)Show SMILES Cc1cc(ccc1S(=O)(=O)N(CCO)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50148889

(CHEMBL121896 | [3-(2,4-Dimethoxy-phenyl)-2,5-dimet...)Show SMILES CCCN(CCOC)c1cc(C)nc2c(c(C)nn12)-c1ccc(OC)cc1OC |(12.65,-5.99,;12.6,-7.53,;13.92,-8.34,;13.89,-9.88,;15.19,-10.68,;15.15,-12.22,;16.47,-13.03,;16.45,-14.57,;12.53,-10.61,;12.48,-12.15,;11.11,-12.9,;11.06,-14.42,;9.8,-12.09,;9.85,-10.53,;8.75,-9.45,;9.41,-8.08,;8.68,-6.72,;10.95,-8.29,;11.22,-9.8,;7.23,-9.73,;6.72,-11.18,;5.21,-11.45,;4.21,-10.28,;2.69,-10.55,;2.18,-11.99,;4.72,-8.83,;6.24,-8.55,;6.77,-7.1,;5.79,-5.93,)| Show InChI InChI=1S/C22H30N4O3/c1-7-10-25(11-12-27-4)20-13-15(2)23-22-21(16(3)24-26(20)22)18-9-8-17(28-5)14-19(18)29-6/h8-9,13-14H,7,10-12H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50071235
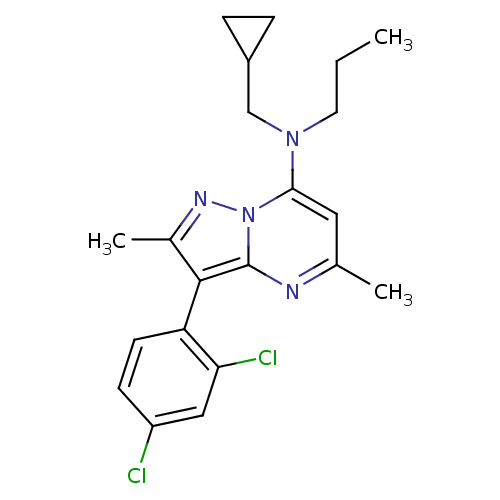
(CHEMBL65078 | Cyclopropylmethyl-[3-(2,4-dichloro-p...)Show SMILES CCCN(CC1CC1)c1cc(C)nc2c(c(C)nn12)-c1ccc(Cl)cc1Cl |(17.18,-6.55,;17.15,-8.09,;15.79,-8.82,;15.75,-10.36,;17.06,-11.17,;17.01,-12.71,;18.31,-13.5,;15.65,-13.44,;14.4,-11.1,;14.35,-12.63,;12.98,-13.38,;12.93,-14.91,;11.67,-12.57,;11.72,-11.01,;10.61,-9.94,;11.28,-8.56,;10.55,-7.21,;12.81,-8.78,;13.09,-10.29,;9.11,-10.22,;8.1,-9.04,;6.59,-9.31,;6.08,-10.76,;4.57,-11.03,;7.07,-11.93,;8.59,-11.67,;9.57,-12.83,)| Show InChI InChI=1S/C21H24Cl2N4/c1-4-9-26(12-15-5-6-15)19-10-13(2)24-21-20(14(3)25-27(19)21)17-8-7-16(22)11-18(17)23/h7-8,10-11,15H,4-6,9,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50593490
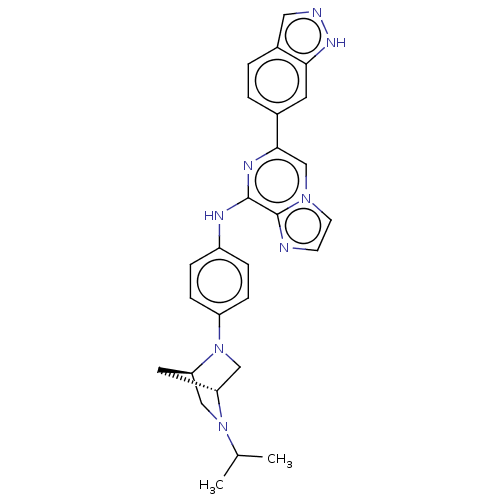
(CHEMBL5174884)Show SMILES [H][C@@]12CN(c3ccc(Nc4nc(cn5ccnc45)-c4ccc5cn[nH]c5c4)cc3)[C@@]([H])(CN1C(C)C)C2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM383753
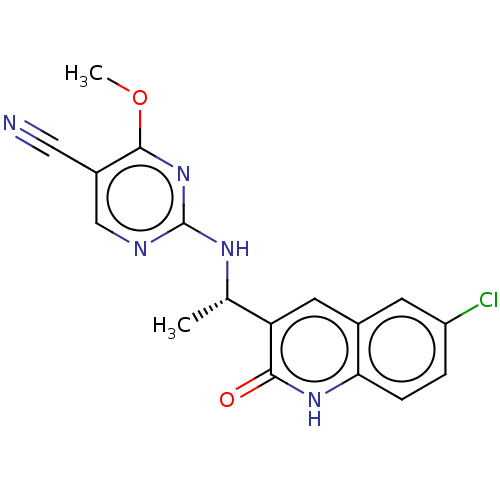
(US10280150, Cmpd No I-36 | US10550099, Compound I-...)Show SMILES COc1nc(N[C@@H](C)c2cc3cc(Cl)ccc3[nH]c2=O)ncc1C#N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM377595
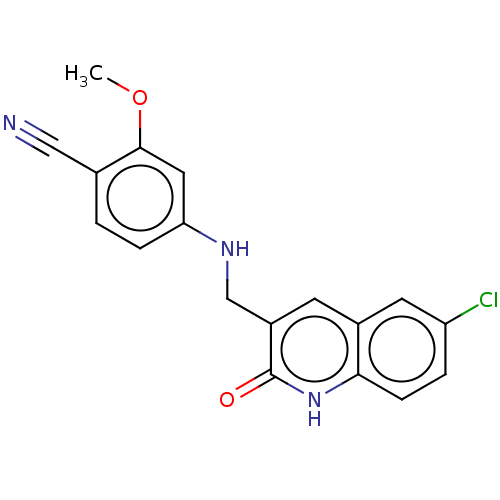
(4-{[(6-chloro-2-oxo-1,2- | US10266495, Compound I-...)Show InChI InChI=1S/C18H14ClN3O2/c1-24-17-8-15(4-2-11(17)9-20)21-10-13-6-12-7-14(19)3-5-16(12)22-18(13)23/h2-8,21H,10H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50435164
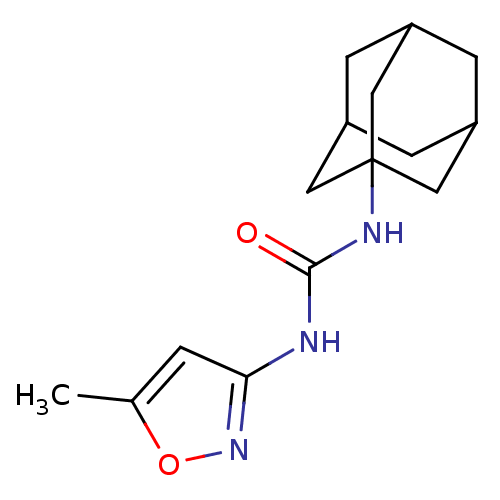
(CHEMBL2392756)Show SMILES Cc1cc(NC(=O)NC23CC4CC(CC(C4)C2)C3)no1 |TLB:7:8:11:15.14.13,THB:9:10:13:17.8.16,9:8:11.10.15:13,16:8:11:15.14.13,16:14:11:17.9.8| Show InChI InChI=1S/C15H21N3O2/c1-9-2-13(18-20-9)16-14(19)17-15-6-10-3-11(7-15)5-12(4-10)8-15/h2,10-12H,3-8H2,1H3,(H2,16,17,18,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50133388

(CHEMBL3632824)Show SMILES NC(=O)c1cc(F)ccc1-c1ccc(CSc2nnc(o2)-c2ccc3OCCOc3c2)cc1 Show InChI InChI=1S/C24H18FN3O4S/c25-17-6-7-18(19(12-17)22(26)29)15-3-1-14(2-4-15)13-33-24-28-27-23(32-24)16-5-8-20-21(11-16)31-10-9-30-20/h1-8,11-12H,9-10,13H2,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50389083
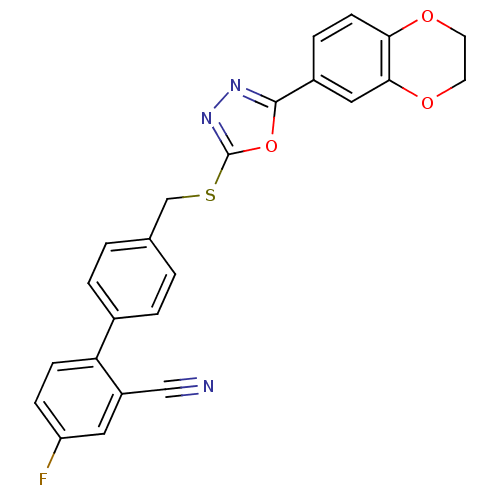
(CHEMBL2064535)Show SMILES Fc1ccc(-c2ccc(CSc3nnc(o3)-c3ccc4OCCOc4c3)cc2)c(c1)C#N Show InChI InChI=1S/C24H16FN3O3S/c25-19-6-7-20(18(11-19)13-26)16-3-1-15(2-4-16)14-32-24-28-27-23(31-24)17-5-8-21-22(12-17)30-10-9-29-21/h1-8,11-12H,9-10,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |
O43924/P16499/P18545/P35913/P51160/Q13956
(Homo sapiens (Human)) | BDBM50428976

(CHEMBL2333219)Show SMILES COc1ccc(CNc2c(CO)cnc3c(cc(cc23)C#N)C2CC2)cc1Cl Show InChI InChI=1S/C22H20ClN3O2/c1-28-20-5-2-13(8-19(20)23)10-25-21-16(12-27)11-26-22-17(15-3-4-15)6-14(9-24)7-18(21)22/h2,5-8,11,15,27H,3-4,10,12H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116614
BindingDB Entry DOI: 10.7270/Q21Z48DS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data