 Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50018720
Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50018720 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104689
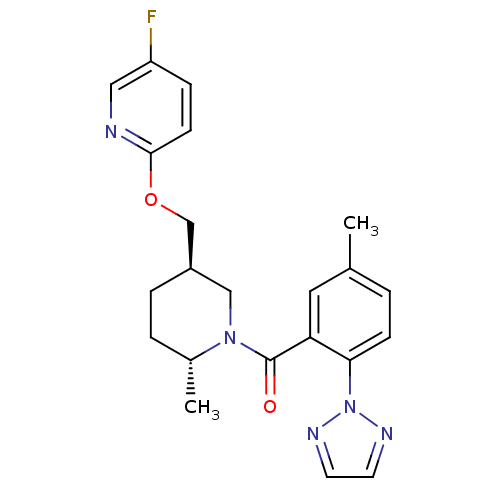
(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692
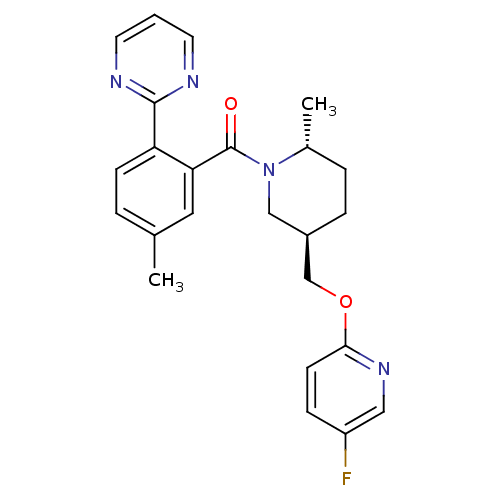
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50088381

(ADL 8-2698 | Alvimopan | Entereg)Show SMILES C[C@H]1CN(C[C@H](Cc2ccccc2)C(=O)NCC(O)=O)CC[C@@]1(C)c1cccc(O)c1 Show InChI InChI=1S/C25H32N2O4/c1-18-16-27(12-11-25(18,2)21-9-6-10-22(28)14-21)17-20(24(31)26-15-23(29)30)13-19-7-4-3-5-8-19/h3-10,14,18,20,28H,11-13,15-17H2,1-2H3,(H,26,31)(H,29,30)/t18-,20-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104689

(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50092810
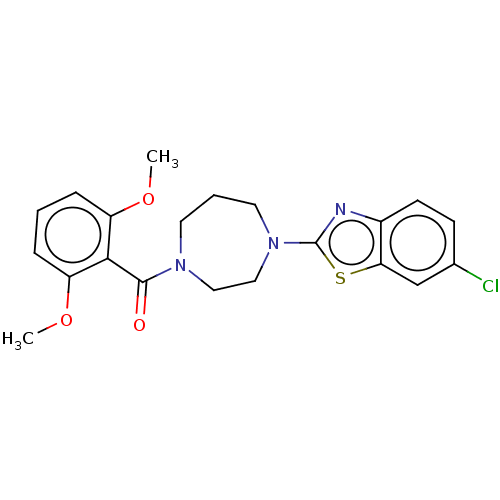
(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50611825

(CHEMBL5273790)Show SMILES Cc1ccc(c(c1)C(=O)N1CCC[C@@H](COc2ccc(F)cn2)C1)-n1nccn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50045772
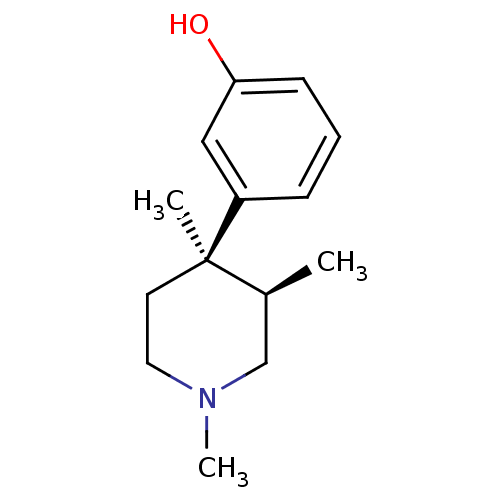
(3-((3R,4R)-1,3,4-Trimethyl-piperidin-4-yl)-phenol ...)Show InChI InChI=1S/C14H21NO/c1-11-10-15(3)8-7-14(11,2)12-5-4-6-13(16)9-12/h4-6,9,11,16H,7-8,10H2,1-3H3/t11-,14+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50611825

(CHEMBL5273790)Show SMILES Cc1ccc(c(c1)C(=O)N1CCC[C@@H](COc2ccc(F)cn2)C1)-n1nccn1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2A
(Homo sapiens (Human)) | BDBM50611824

(CHEMBL118755)Show SMILES Clc1ccc2c(c1)[nH]c1c(c(=O)n(Cc3ccncc3)[nH]c1=O)c2=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50092810

(CHEMBL3586412)Show SMILES COc1cccc(OC)c1C(=O)N1CCCN(CC1)c1nc2ccc(Cl)cc2s1 Show InChI InChI=1S/C21H22ClN3O3S/c1-27-16-5-3-6-17(28-2)19(16)20(26)24-9-4-10-25(12-11-24)21-23-15-8-7-14(22)13-18(15)29-21/h3,5-8,13H,4,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2A
(Homo sapiens (Human)) | BDBM50611823
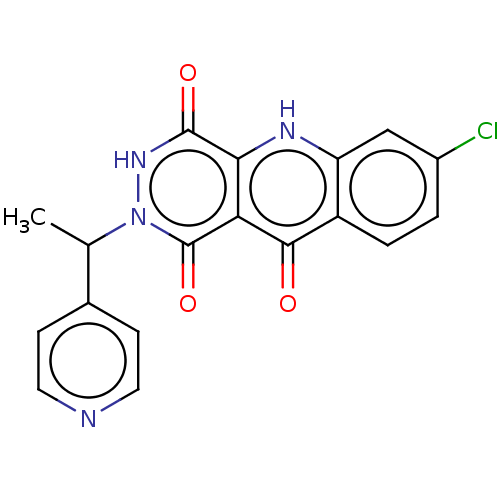
(CHEMBL1184118)Show SMILES CC(c1ccncc1)n1[nH]c(=O)c2[nH]c3cc(Cl)ccc3c(=O)c2c1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067933
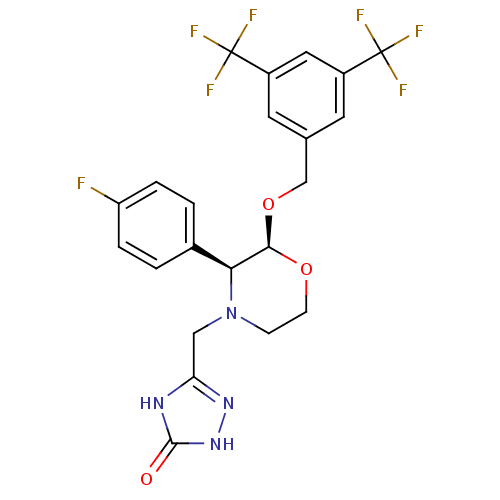
(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES Fc1ccc(cc1)[C@H]1[C@@H](OCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)OCCN1Cc1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C22H19F7N4O3/c23-16-3-1-13(2-4-16)18-19(35-6-5-33(18)10-17-30-20(34)32-31-17)36-11-12-7-14(21(24,25)26)9-15(8-12)22(27,28)29/h1-4,7-9,18-19H,5-6,10-11H2,(H2,30,31,32,34)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136
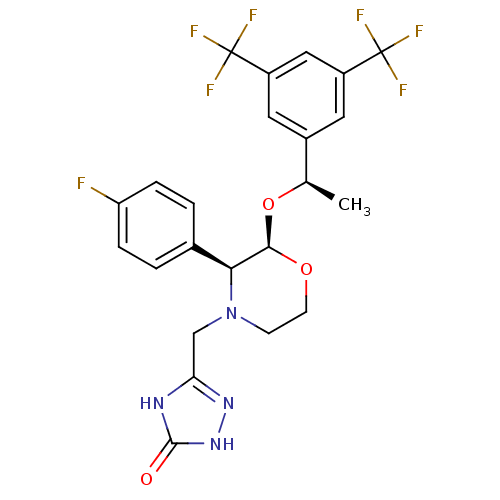
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM34168

(LOVASTATIN | MLS000069585 | SMR000058779 | US91151...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein
(Mus musculus) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Mus musculus (mouse)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Breakpoint cluster region protein
(Mus musculus) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Mus musculus (mouse)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit gamma
(Homo sapiens (Human)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50290422

(4-(4-Methyl-piperazin-1-ylmethyl)-N-[3-(4-pyridin-...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2cccc(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C28H29N7O/c1-34-14-16-35(17-15-34)20-21-7-9-22(10-8-21)27(36)31-24-5-2-6-25(18-24)32-28-30-13-11-26(33-28)23-4-3-12-29-19-23/h2-13,18-19H,14-17,20H2,1H3,(H,31,36)(H,30,32,33) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data