 Found 43 hits Enz. Inhib. hit(s) with all data for entry = 50018772
Found 43 hits Enz. Inhib. hit(s) with all data for entry = 50018772 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546436
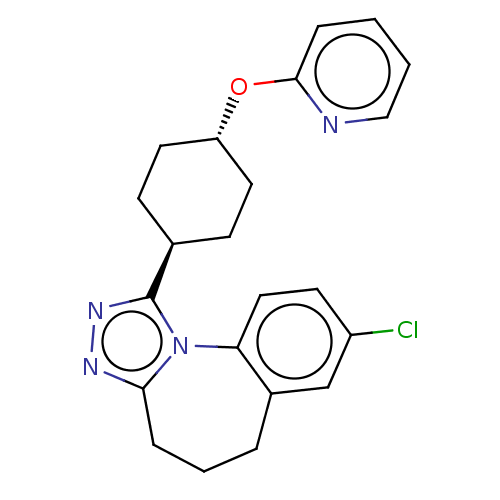
(CHEMBL4799793)Show SMILES Clc1ccc-2c(CCCc3nnc([C@H]4CC[C@@H](CC4)Oc4ccccn4)n-23)c1 |r,wU:13.12,wD:16.19,(20.56,-28.4,;21.91,-29.12,;23.23,-28.31,;24.58,-29.05,;24.62,-30.59,;23.31,-31.39,;23.13,-32.92,;24.2,-34.02,;25.73,-33.86,;26.56,-32.56,;28.09,-32.56,;28.56,-31.1,;27.32,-30.2,;27.31,-28.66,;25.98,-27.88,;25.99,-26.34,;27.34,-25.57,;28.67,-26.35,;28.66,-27.9,;27.34,-24.04,;26.01,-23.27,;26.02,-21.74,;24.69,-20.97,;23.36,-21.74,;23.36,-23.28,;24.69,-24.04,;26.07,-31.1,;21.96,-30.66,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50546428
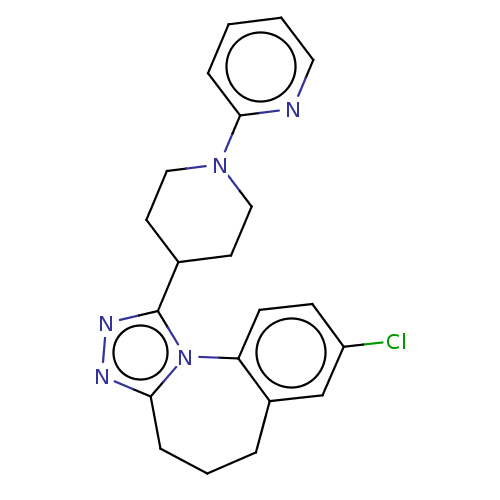
(CHEMBL4778839)Show SMILES Clc1ccc-2c(CCCc3nnc(C4CCN(CC4)c4ccccn4)n-23)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50612387
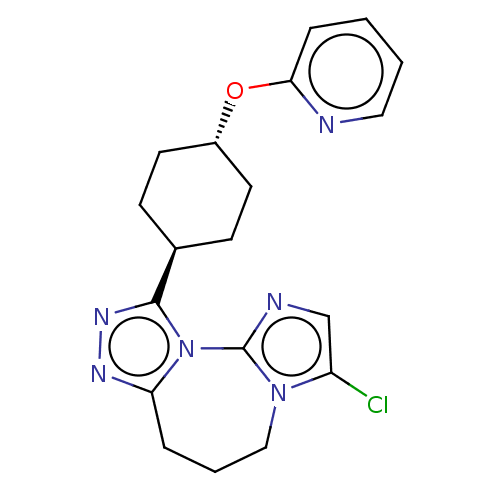
(CHEMBL5283016)Show SMILES Clc1cnc-2n1CCCc1nnc([C@H]3CC[C@@H](CC3)Oc3ccccn3)n-21 |r,wU:13.13,wD:16.20,(.89,-4.68,;1.29,-3.19,;.26,-2.08,;1.03,-.72,;2.55,-1,;2.74,-2.55,;4.05,-3.36,;5.52,-2.83,;6.02,-1.37,;5.18,-.06,;5.8,1.31,;4.65,2.37,;3.31,1.6,;1.98,2.37,;.65,1.6,;-.69,2.37,;-.69,3.91,;.65,4.68,;1.98,3.91,;-2.02,4.68,;-3.35,3.91,;-4.68,4.68,;-6.02,3.91,;-6.02,2.37,;-4.68,1.6,;-3.35,2.37,;3.65,.11,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50036835
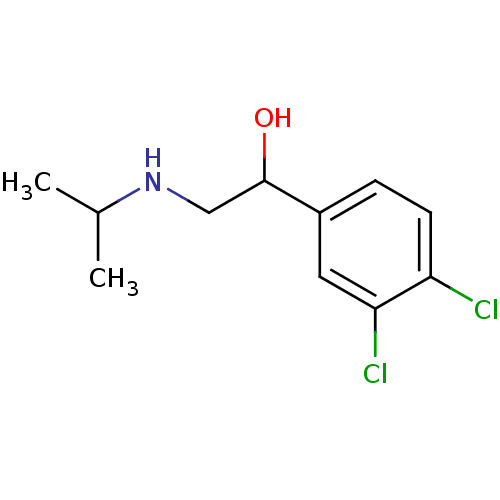
(1-(3,4-Dichloro-phenyl)-2-isopropylamino-ethanol |...)Show InChI InChI=1S/C11H15Cl2NO/c1-7(2)14-6-11(15)8-3-4-9(12)10(13)5-8/h3-5,7,11,14-15H,6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polymerase acidic protein
(Hepatitis C virus) | BDBM50385503
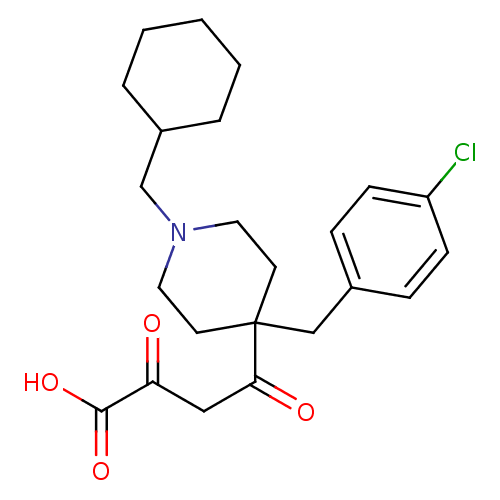
(CHEMBL2040554)Show SMILES OC(=O)C(=O)CC(=O)C1(Cc2ccc(Cl)cc2)CCN(CC2CCCCC2)CC1 Show InChI InChI=1S/C23H30ClNO4/c24-19-8-6-17(7-9-19)15-23(21(27)14-20(26)22(28)29)10-12-25(13-11-23)16-18-4-2-1-3-5-18/h6-9,18H,1-5,10-16H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polymerase acidic protein
(Hepatitis C virus) | BDBM50612374
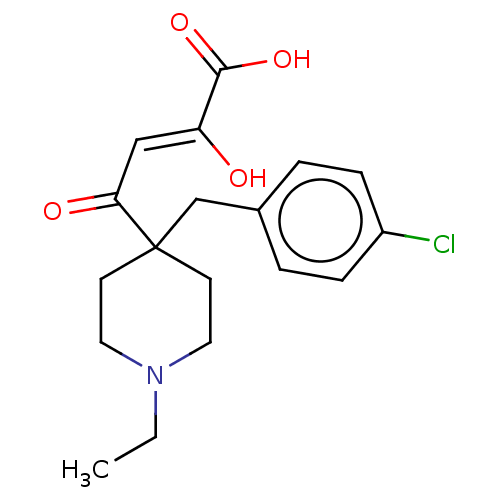
(CHEMBL5286364)Show SMILES CCN1CCC(Cc2ccc(Cl)cc2)(CC1)C(=O)\C=C(/O)C(O)=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(BOVINE) | BDBM50036835

(1-(3,4-Dichloro-phenyl)-2-isopropylamino-ethanol |...)Show InChI InChI=1S/C11H15Cl2NO/c1-7(2)14-6-11(15)8-3-4-9(12)10(13)5-8/h3-5,7,11,14-15H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50612388

(CHEMBL5283584)Show SMILES Clc1cnc-2n1CCCc1nnc(C3CCN(CC3)c3ccccn3)n-21 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50036840

(CHEMBL38033 | Propionaldehyde O-(3-tert-butylamino...)Show InChI InChI=1S/C10H22N2O2/c1-5-6-12-14-8-9(13)7-11-10(2,3)4/h6,9,11,13H,5,7-8H2,1-4H3/b12-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(BOVINE) | BDBM50036841
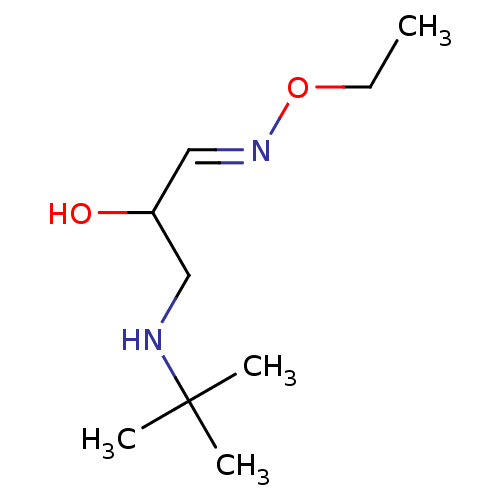
(3-tert-Butylamino-2-hydroxy-propionaldehyde O-ethy...)Show InChI InChI=1S/C9H20N2O2/c1-5-13-11-7-8(12)6-10-9(2,3)4/h7-8,10,12H,5-6H2,1-4H3/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50036841

(3-tert-Butylamino-2-hydroxy-propionaldehyde O-ethy...)Show InChI InChI=1S/C9H20N2O2/c1-5-13-11-7-8(12)6-10-9(2,3)4/h7-8,10,12H,5-6H2,1-4H3/b11-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(BOVINE) | BDBM50036840

(CHEMBL38033 | Propionaldehyde O-(3-tert-butylamino...)Show InChI InChI=1S/C10H22N2O2/c1-5-6-12-14-8-9(13)7-11-10(2,3)4/h6,9,11,13H,5,7-8H2,1-4H3/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20557
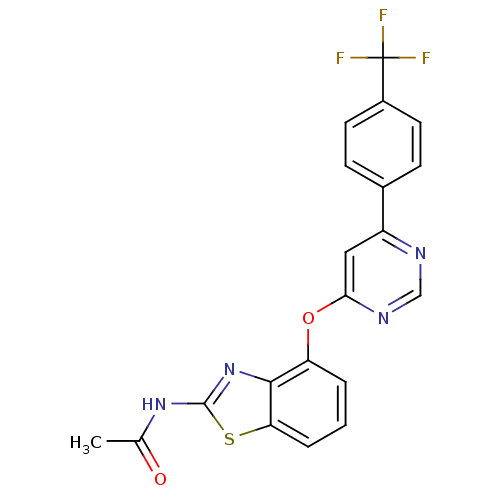
(AMG 517 | CHEMBL229430 | JMC503515 Compound 23 | N...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)-c3ccc(cc3)C(F)(F)F)cccc2s1 Show InChI InChI=1S/C20H13F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-10H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20581
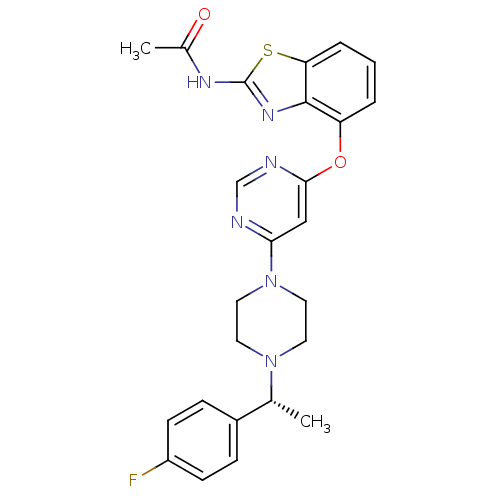
(N-{4-[(6-{4-[(1R)-1-(4-fluorophenyl)ethyl]piperazi...)Show SMILES C[C@@H](N1CCN(CC1)c1cc(Oc2cccc3sc(NC(C)=O)nc23)ncn1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H25FN6O2S/c1-16(18-6-8-19(26)9-7-18)31-10-12-32(13-11-31)22-14-23(28-15-27-22)34-20-4-3-5-21-24(20)30-25(35-21)29-17(2)33/h3-9,14-16H,10-13H2,1-2H3,(H,29,30,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20561
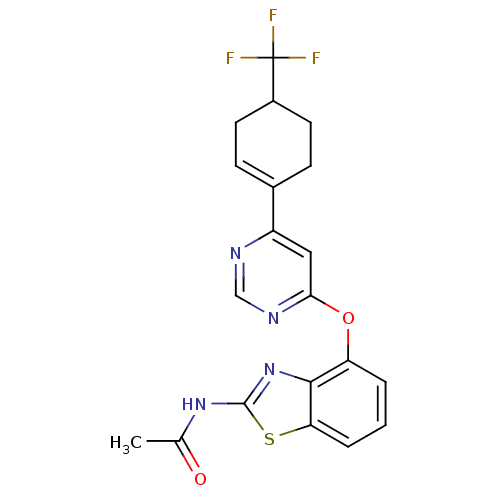
(N-[4-({6-[4-(trifluoromethyl)cyclohex-1-en-1-yl]py...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)C3=CCC(CC3)C(F)(F)F)cccc2s1 |t:16| Show InChI InChI=1S/C20H17F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-5,9-10,13H,6-8H2,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50132080
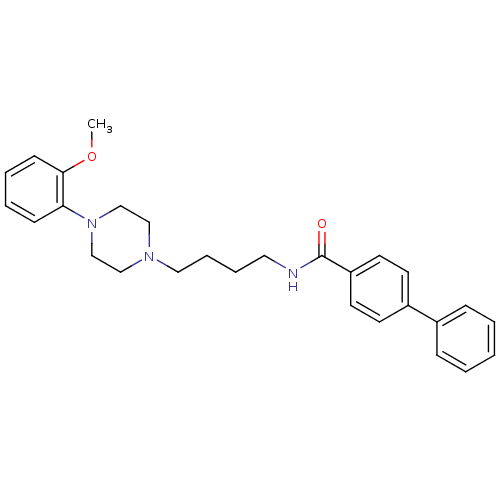
(Biphenyl-4-carboxylic acid {4-[4-(2-methoxy-phenyl...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C28H33N3O2/c1-33-27-12-6-5-11-26(27)31-21-19-30(20-22-31)18-8-7-17-29-28(32)25-15-13-24(14-16-25)23-9-3-2-4-10-23/h2-6,9-16H,7-8,17-22H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50612384
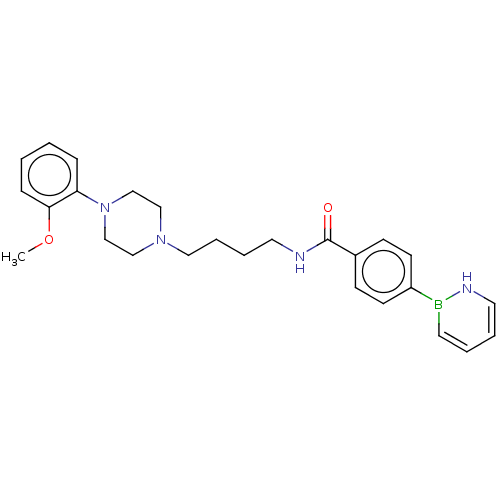
(CHEMBL5281608)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)B2NC=CC=C2)CC1 |c:29,31| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20563

(N-[4-({6-[4-(trifluoromethyl)piperidin-1-yl]pyrimi...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)N3CCC(CC3)C(F)(F)F)cccc2s1 Show InChI InChI=1S/C19H18F3N5O2S/c1-11(28)25-18-26-17-13(3-2-4-14(17)30-18)29-16-9-15(23-10-24-16)27-7-5-12(6-8-27)19(20,21)22/h2-4,9-10,12H,5-8H2,1H3,(H,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20562
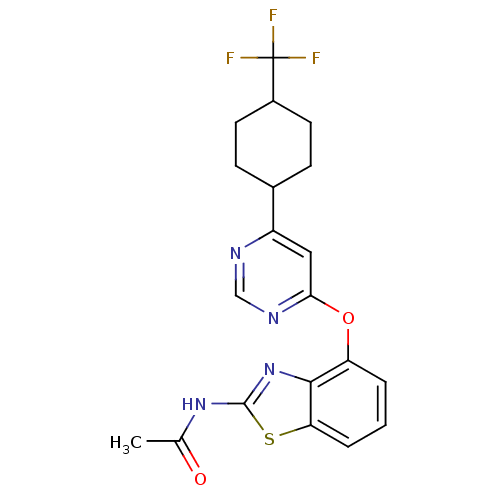
(N-[4-({6-[4-(trifluoromethyl)cyclohexyl]pyrimidin-...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)C3CCC(CC3)C(F)(F)F)cccc2s1 |(15.15,-10.72,;16.44,-11.49,;16.44,-13.04,;17.8,-10.72,;19.09,-11.49,;19.28,-12.98,;20.77,-13.37,;21.61,-14.66,;20.83,-16.01,;19.28,-16.01,;18.57,-17.37,;17.02,-17.5,;16.25,-16.08,;16.96,-14.79,;18.51,-14.72,;16.31,-18.79,;17.15,-20.08,;16.31,-21.44,;14.83,-21.5,;13.99,-20.14,;14.83,-18.79,;14.12,-22.86,;12.76,-23.63,;15.47,-23.57,;12.76,-22.08,;23.09,-14.66,;23.87,-13.3,;23.16,-12.01,;21.61,-12.01,;20.51,-10.91,)| Show InChI InChI=1S/C20H19F3N4O2S/c1-11(28)26-19-27-18-15(3-2-4-16(18)30-19)29-17-9-14(24-10-25-17)12-5-7-13(8-6-12)20(21,22)23/h2-4,9-10,12-13H,5-8H2,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM13530

(4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...)Show SMILES CN1CCN(Cc2ccc(cc2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Rattus norvegicus (rat)) | BDBM20569

(N-[4-({6-[4-(2,2,2-trifluoroethyl)piperazin-1-yl]p...)Show SMILES CC(=O)Nc1nc2c(Oc3cc(ncn3)N3CCN(CC(F)(F)F)CC3)cccc2s1 Show InChI InChI=1S/C19H19F3N6O2S/c1-12(29)25-18-26-17-13(3-2-4-14(17)31-18)30-16-9-15(23-11-24-16)28-7-5-27(6-8-28)10-19(20,21)22/h2-4,9,11H,5-8,10H2,1H3,(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612380

(CHEMBL5271953)Show SMILES CN1CCN(C[C@H]2C[C@@H]2C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612376
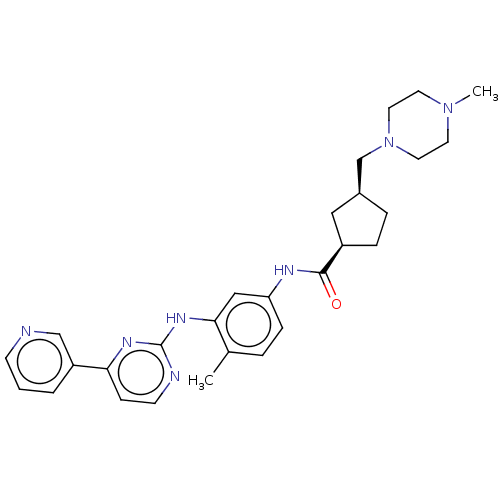
(CHEMBL5275489)Show SMILES CN1CCN(C[C@H]2CC[C@H](C2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612375
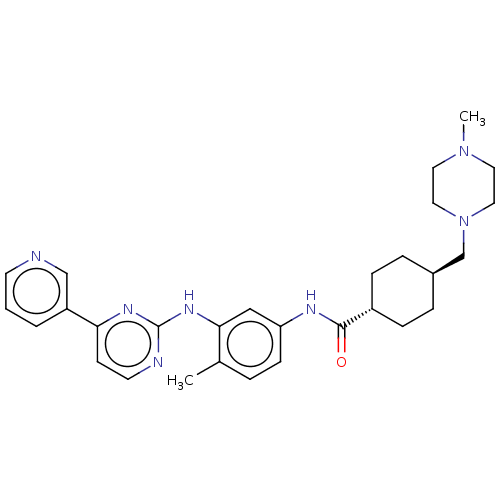
(CHEMBL5287588)Show SMILES CN1CCN(C[C@H]2CC[C@@H](CC2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r,wU:9.12,wD:6.5,(-10.84,3.85,;-10.04,2.53,;-10.78,1.18,;-9.98,-.1,;-8.44,-.1,;-7.64,-1.38,;-6.1,-1.38,;-5.3,-2.7,;-3.76,-2.7,;-3.02,-1.32,;-3.82,0,;-5.36,0,;-1.48,-1.32,;-.71,-2.62,;-.74,.1,;.8,.1,;1.54,1.49,;3.08,1.49,;3.88,.17,;5.42,.17,;3.14,-1.18,;3.91,-2.51,;5.45,-2.51,;6.23,-3.85,;7.76,-3.85,;8.53,-2.51,;7.76,-1.18,;6.22,-1.18,;8.53,.16,;10.08,.16,;10.84,1.49,;10.07,2.82,;8.53,2.82,;7.76,1.49,;1.6,-1.18,;-7.7,1.25,;-8.5,2.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612381
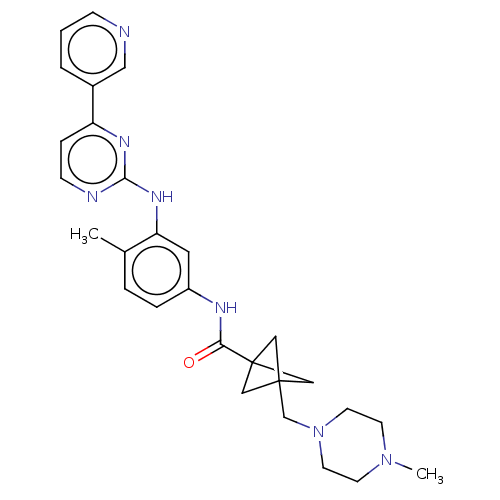
(CHEMBL5279557)Show SMILES CN1CCN(CC23CC(C2)(C3)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612378

(CHEMBL5282860)Show SMILES CN1CCN(C[C@H]2C[C@@H](C2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r,wU:8.10,wD:6.5,(-10.36,3.78,;-9.56,2.46,;-10.29,1.11,;-9.48,-.16,;-7.94,-.16,;-7.14,-1.43,;-5.6,-1.43,;-4.48,-2.49,;-3.42,-1.37,;-4.54,-.31,;-1.88,-1.37,;-1.07,-2.64,;-1.15,.07,;.39,.07,;1.13,1.47,;2.67,1.47,;3.47,.16,;5.01,.16,;2.74,-1.2,;3.55,-2.46,;5.09,-2.46,;5.89,-3.78,;7.43,-3.78,;8.17,-2.38,;7.36,-1.07,;5.82,-1.07,;8.09,.29,;9.63,.29,;10.36,1.69,;9.56,3,;8.02,3,;7.28,1.6,;1.2,-1.2,;-7.21,1.2,;-8.02,2.46,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612383

(CHEMBL5266376)Show SMILES CN1CCN(CCCCCC(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612379
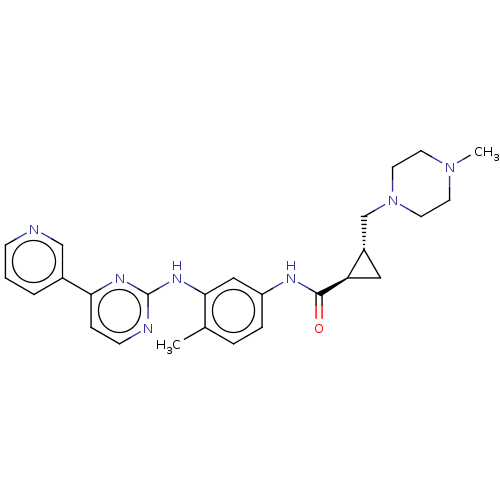
(CHEMBL5281225)Show SMILES CN1CCN(C[C@@H]2C[C@H]2C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612377

(CHEMBL5273946)Show SMILES CN1CCN(C[C@H]2C[C@H](C2)C(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 |r,wU:6.5,8.10,(-10.36,3.78,;-9.56,2.46,;-10.29,1.11,;-9.48,-.16,;-7.94,-.16,;-7.14,-1.43,;-5.6,-1.43,;-4.54,-.31,;-3.42,-1.37,;-4.48,-2.49,;-1.88,-1.37,;-1.07,-2.64,;-1.15,.07,;.39,.07,;1.13,1.47,;2.67,1.47,;3.47,.16,;5.01,.16,;2.74,-1.2,;3.55,-2.46,;5.09,-2.46,;5.89,-3.78,;7.43,-3.78,;8.17,-2.38,;7.36,-1.07,;5.82,-1.07,;8.09,.29,;9.63,.29,;10.36,1.69,;9.56,3,;8.02,3,;7.28,1.6,;1.2,-1.2,;-7.21,1.2,;-8.02,2.46,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50612382
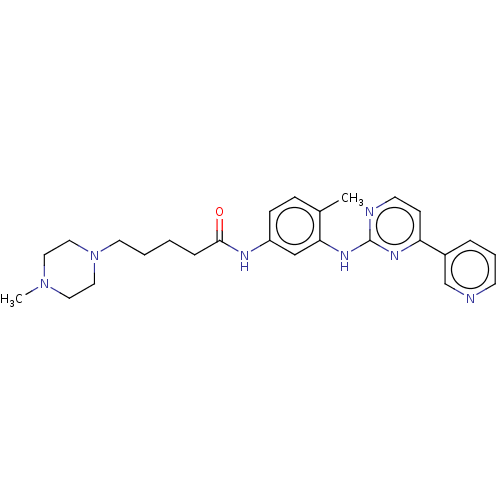
(CHEMBL5269948)Show SMILES CN1CCN(CCCCC(=O)Nc2ccc(C)c(Nc3nccc(n3)-c3cccnc3)c2)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50612384

(CHEMBL5281608)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)B2NC=CC=C2)CC1 |c:29,31| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50589218
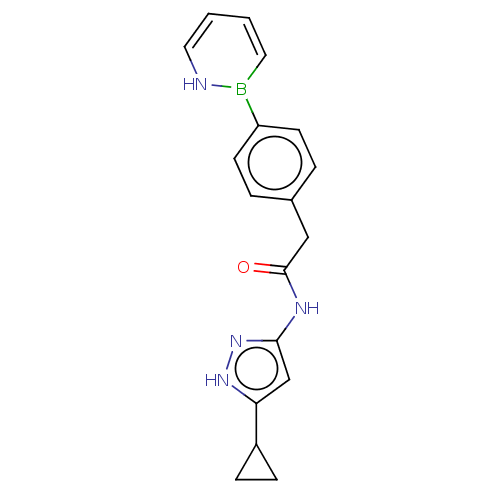
(CHEMBL5200108)Show SMILES O=C(Cc1ccc(cc1)B1NC=CC=C1)Nc1cc([nH]n1)C1CC1 |c:12,14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50612386
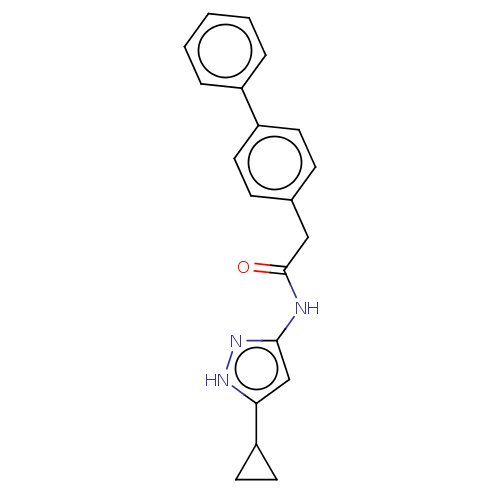
(CHEMBL115933)Show SMILES O=C(Cc1ccc(cc1)-c1ccccc1)Nc1cc([nH]n1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50415442

(CHEMBL598609)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H17F3N2O3S/c22-21(23,24)18-10-11-19(26-14-18)30(28,29)13-12-25-20(27)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-11,14H,12-13H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50132080

(Biphenyl-4-carboxylic acid {4-[4-(2-methoxy-phenyl...)Show SMILES COc1ccccc1N1CCN(CCCCNC(=O)c2ccc(cc2)-c2ccccc2)CC1 Show InChI InChI=1S/C28H33N3O2/c1-33-27-12-6-5-11-26(27)31-21-19-30(20-22-31)18-8-7-17-29-28(32)25-15-13-24(14-16-25)23-9-3-2-4-10-23/h2-6,9-16H,7-8,17-22H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50612385

(CHEMBL5289407)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(cc1)B1NC=CC=C1 |c:28,30| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50052256
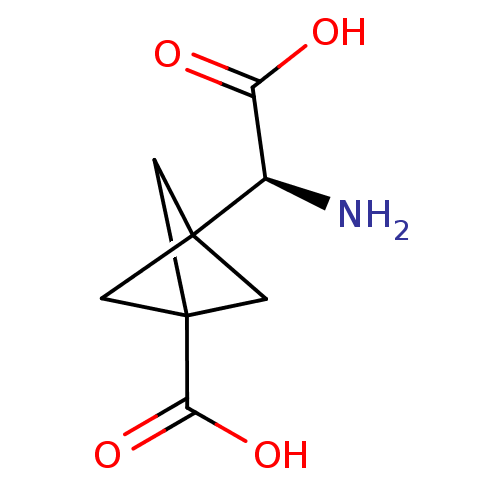
((S)-3-(amino(carboxy)methyl)bicyclo[1.1.1]pentane-...)Show InChI InChI=1S/C8H11NO4/c9-4(5(10)11)7-1-8(2-7,3-7)6(12)13/h4H,1-3,9H2,(H,10,11)(H,12,13)/t4-,7?,8?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50612385

(CHEMBL5289407)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(cc1)B1NC=CC=C1 |c:28,30| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50612386

(CHEMBL115933)Show SMILES O=C(Cc1ccc(cc1)-c1ccccc1)Nc1cc([nH]n1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50589218

(CHEMBL5200108)Show SMILES O=C(Cc1ccc(cc1)B1NC=CC=C1)Nc1cc([nH]n1)C1CC1 |c:12,14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50415442

(CHEMBL598609)Show SMILES FC(F)(F)c1ccc(nc1)S(=O)(=O)CCNC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C21H17F3N2O3S/c22-21(23,24)18-10-11-19(26-14-18)30(28,29)13-12-25-20(27)17-8-6-16(7-9-17)15-4-2-1-3-5-15/h1-11,14H,12-13H2,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50053588
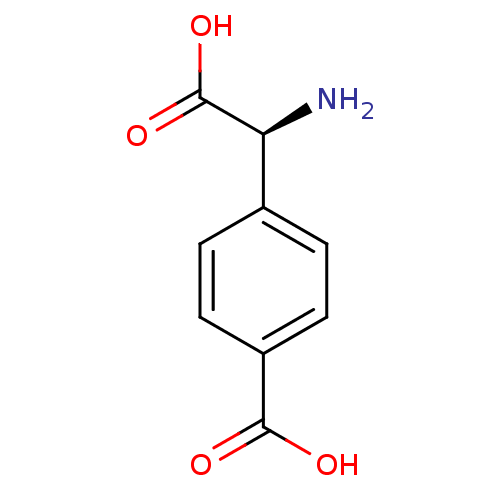
((S)-4-(amino(carboxy)methyl)benzoic acid | (S)-4-c...)Show InChI InChI=1S/C9H9NO4/c10-7(9(13)14)5-1-3-6(4-2-5)8(11)12/h1-4,7H,10H2,(H,11,12)(H,13,14)/t7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50400857

(CHEMBL2204334)Show InChI InChI=1S/C8H11N5O2/c9-4(5(14)15)7-1-8(2-7,3-7)6-10-12-13-11-6/h4H,1-3,9H2,(H,14,15)(H,10,11,12,13)/t4-,7?,8?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 6.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data