 Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50028590
Found 24 hits Enz. Inhib. hit(s) with all data for entry = 50028590 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50276347
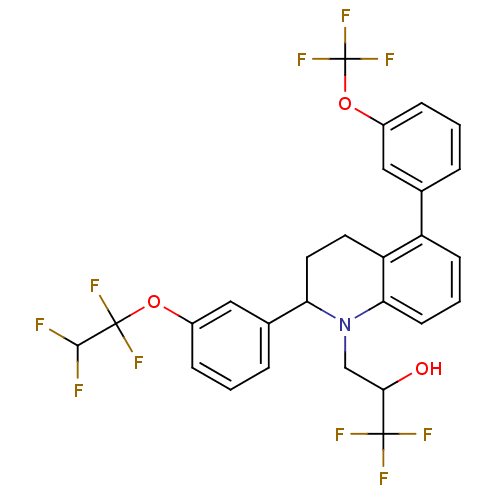
((+/-)-1,1,1-trifluoro-3-(2-(3-(1,1,2,2-tetrafluoro...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C27H21F10NO3/c28-24(29)26(33,34)40-17-6-2-5-16(13-17)21-11-10-20-19(15-4-1-7-18(12-15)41-27(35,36)37)8-3-9-22(20)38(21)14-23(39)25(30,31)32/h1-9,12-13,21,23-24,39H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50276347

((+/-)-1,1,1-trifluoro-3-(2-(3-(1,1,2,2-tetrafluoro...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C27H21F10NO3/c28-24(29)26(33,34)40-17-6-2-5-16(13-17)21-11-10-20-19(15-4-1-7-18(12-15)41-27(35,36)37)8-3-9-22(20)38(21)14-23(39)25(30,31)32/h1-9,12-13,21,23-24,39H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277885
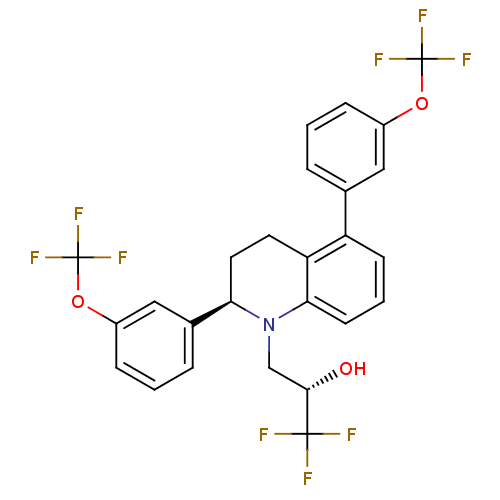
(CHEMBL490868 | Tetrahydroquinline A)Show SMILES O[C@@H](CN1[C@H](CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1)C(F)(F)F |r| Show InChI InChI=1S/C26H20F9NO3/c27-24(28,29)23(37)14-36-21(16-5-2-7-18(13-16)39-26(33,34)35)11-10-20-19(8-3-9-22(20)36)15-4-1-6-17(12-15)38-25(30,31)32/h1-9,12-13,21,23,37H,10-11,14H2/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277959
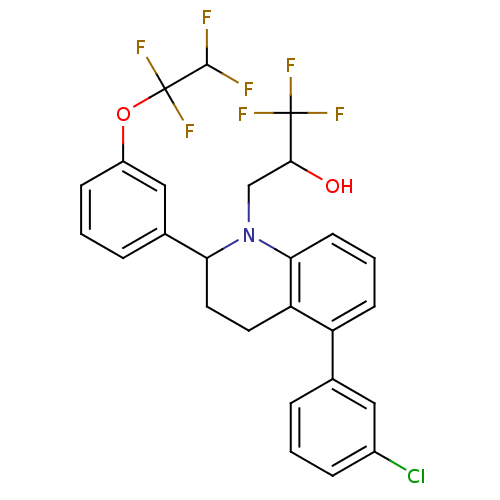
(3-(5-(3-chlorophenyl)-2-(3-(1,1,2,2-te trafluoroet...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(Cl)c1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C26H21ClF7NO2/c27-17-6-1-4-15(12-17)19-8-3-9-22-20(19)10-11-21(35(22)14-23(36)25(30,31)32)16-5-2-7-18(13-16)37-26(33,34)24(28)29/h1-9,12-13,21,23-24,36H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50278012
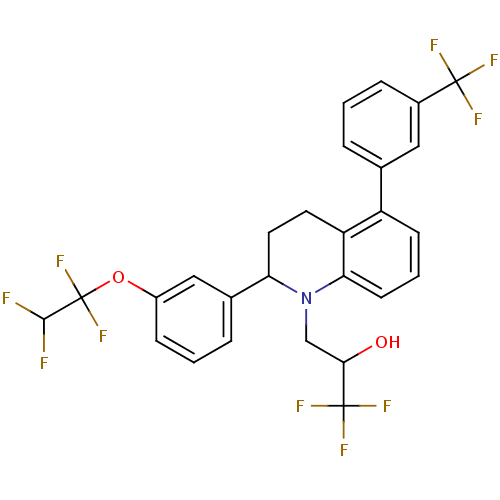
(1,1,1-tri fluoro-3-(2-(3-(1,1,2,2-tetrafluoroethox...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(c1)C(F)(F)F)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C27H21F10NO2/c28-24(29)27(36,37)40-18-7-2-5-16(13-18)21-11-10-20-19(15-4-1-6-17(12-15)25(30,31)32)8-3-9-22(20)38(21)14-23(39)26(33,34)35/h1-9,12-13,21,23-24,39H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50278011
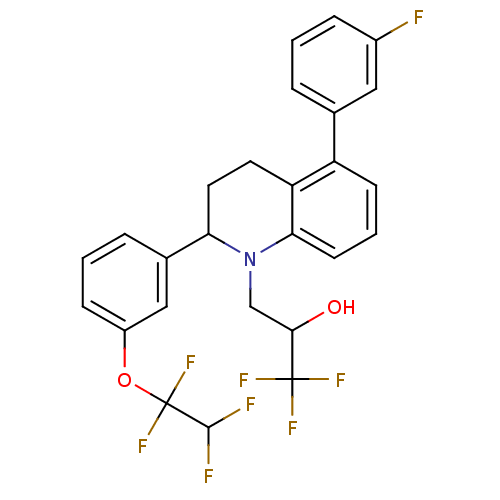
(1,1,1-trifluoro-3-(5-(3-fluorophenyl) -2-(3-(1,1,2...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(F)c1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C26H21F8NO2/c27-17-6-1-4-15(12-17)19-8-3-9-22-20(19)10-11-21(35(22)14-23(36)25(30,31)32)16-5-2-7-18(13-16)37-26(33,34)24(28)29/h1-9,12-13,21,23-24,36H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277884
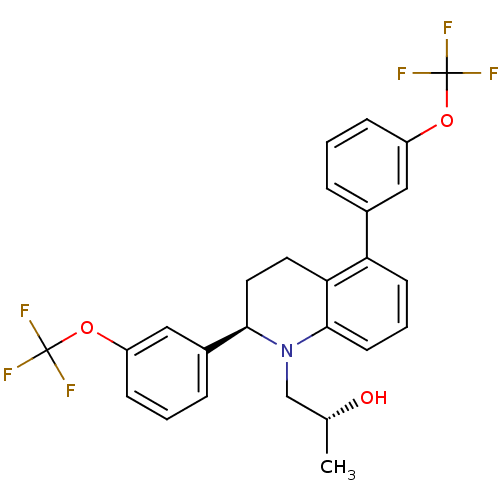
((R)-1-((R)-2,5-bis(3-(trifluoromethoxy)phenyl)-3,4...)Show SMILES C[C@@H](O)CN1[C@H](CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C26H23F6NO3/c1-16(34)15-33-23(18-6-3-8-20(14-18)36-26(30,31)32)12-11-22-21(9-4-10-24(22)33)17-5-2-7-19(13-17)35-25(27,28)29/h2-10,13-14,16,23,34H,11-12,15H2,1H3/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277727

(3-(2,5-bis(3-(trifluoromethoxy) phenyl)-3,4-dihydr...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1)C(F)(F)F Show InChI InChI=1S/C26H20F9NO3/c27-24(28,29)23(37)14-36-21(16-5-2-7-18(13-16)39-26(33,34)35)11-10-20-19(8-3-9-22(20)36)15-4-1-6-17(12-15)38-25(30,31)32/h1-9,12-13,21,23,37H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50278055
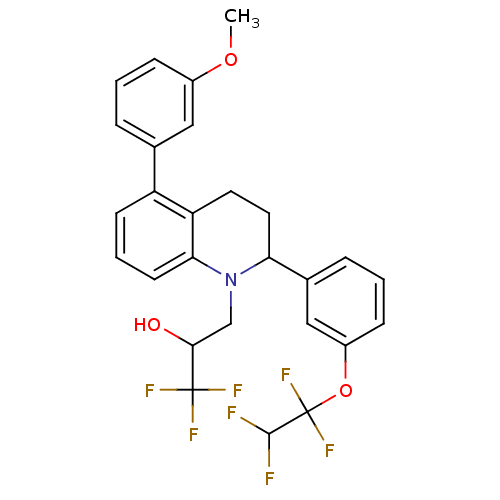
(1,1,1-trifluoro -3-(5-(3-methoxyphenyl)-2-(3-(1,1,...)Show SMILES COc1cccc(c1)-c1cccc2N(CC(O)C(F)(F)F)C(CCc12)c1cccc(OC(F)(F)C(F)F)c1 Show InChI InChI=1S/C27H24F7NO3/c1-37-18-7-2-5-16(13-18)20-9-4-10-23-21(20)11-12-22(35(23)15-24(36)26(30,31)32)17-6-3-8-19(14-17)38-27(33,34)25(28)29/h2-10,13-14,22,24-25,36H,11-12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277688

(1,1,1-trifluoro-3-(5-(3-(trifluoromethoxy)phenyl)-...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H20F9NO2/c27-24(28,29)17-6-1-5-16(12-17)21-11-10-20-19(15-4-2-7-18(13-15)38-26(33,34)35)8-3-9-22(20)36(21)14-23(37)25(30,31)32/h1-9,12-13,21,23,37H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277688

(1,1,1-trifluoro-3-(5-(3-(trifluoromethoxy)phenyl)-...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H20F9NO2/c27-24(28,29)17-6-1-5-16(12-17)21-11-10-20-19(15-4-2-7-18(13-15)38-26(33,34)35)8-3-9-22(20)36(21)14-23(37)25(30,31)32/h1-9,12-13,21,23,37H,10-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277459
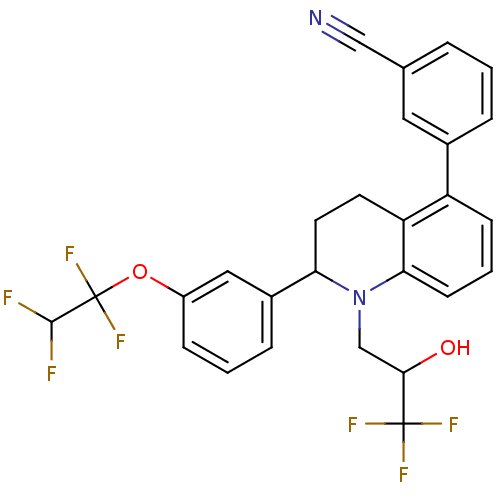
(3-(2-(3-(1,1,2,2-tetrafluoroethoxy)phenyl )-1-(3,3...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(c1)C#N)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C27H21F7N2O2/c28-25(29)27(33,34)38-19-7-2-6-18(13-19)22-11-10-21-20(17-5-1-4-16(12-17)14-35)8-3-9-23(21)36(22)15-24(37)26(30,31)32/h1-9,12-13,22,24-25,37H,10-11,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277812
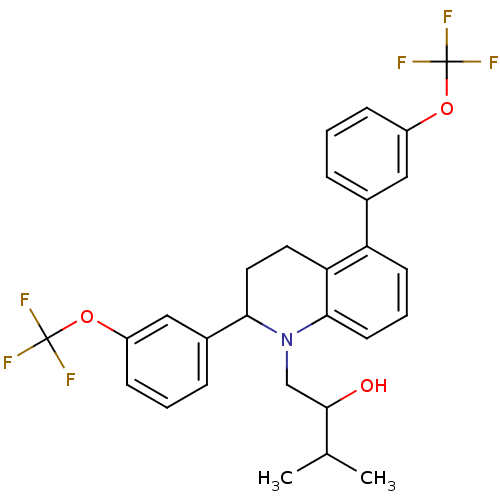
(1-(2,5-bis(3-(trifluoromethoxy) phenyl)-3,4-dihydr...)Show SMILES CC(C)C(O)CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C28H27F6NO3/c1-17(2)26(36)16-35-24(19-7-4-9-21(15-19)38-28(32,33)34)13-12-23-22(10-5-11-25(23)35)18-6-3-8-20(14-18)37-27(29,30)31/h3-11,14-15,17,24,26,36H,12-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277773
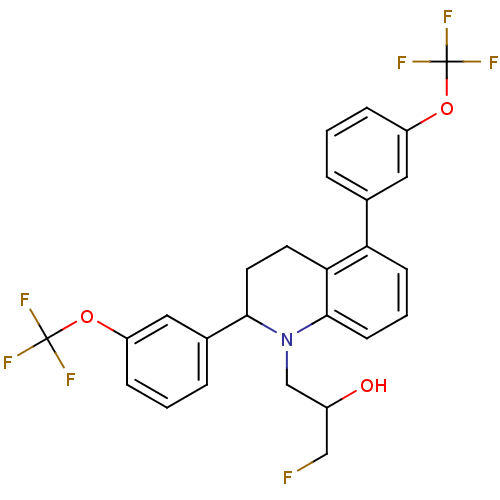
(1-(2,5-bis(3-(trifluoromethoxy)phenyl)-3,4-dihydro...)Show SMILES OC(CF)CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C26H22F7NO3/c27-14-18(35)15-34-23(17-5-2-7-20(13-17)37-26(31,32)33)11-10-22-21(8-3-9-24(22)34)16-4-1-6-19(12-16)36-25(28,29)30/h1-9,12-13,18,23,35H,10-11,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277921

(3-(5-(2,3-dichlorophenyl )-2-(3-(1,1,2,2-tetrafluo...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccc(Cl)c1Cl)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C26H20Cl2F7NO2/c27-19-8-2-7-18(23(19)28)16-6-3-9-21-17(16)10-11-20(36(21)13-22(37)25(31,32)33)14-4-1-5-15(12-14)38-26(34,35)24(29)30/h1-9,12,20,22,24,37H,10-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277460
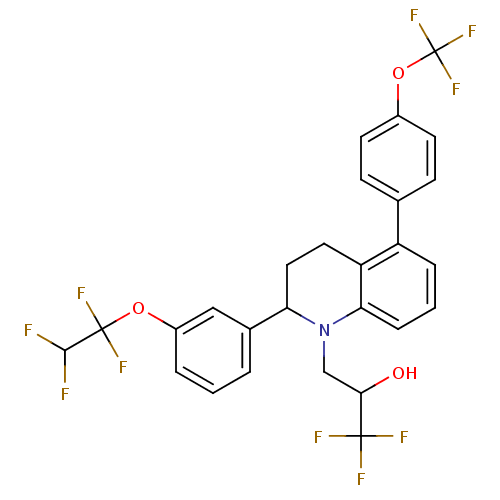
(1,1,1-trifluoro-3-(2-(3-(1,1,2,2-tetrafluoroethoxy...)Show SMILES OC(CN1C(CCc2c1cccc2-c1ccc(OC(F)(F)F)cc1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C27H21F10NO3/c28-24(29)26(33,34)40-18-4-1-3-16(13-18)21-12-11-20-19(15-7-9-17(10-8-15)41-27(35,36)37)5-2-6-22(20)38(21)14-23(39)25(30,31)32/h1-10,13,21,23-24,39H,11-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50276280
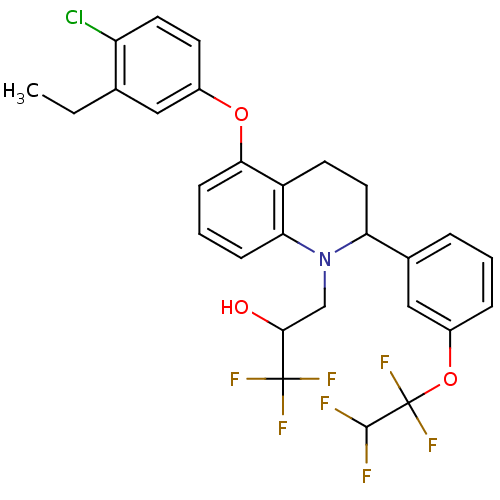
(3-(5-(4-chloro-3-ethylphenoxy)-2 -(3-(1,1,2,2-tetr...)Show SMILES CCc1cc(Oc2cccc3N(CC(O)C(F)(F)F)C(CCc23)c2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C28H25ClF7NO3/c1-2-16-13-18(9-11-21(16)29)39-24-8-4-7-23-20(24)10-12-22(37(23)15-25(38)27(32,33)34)17-5-3-6-19(14-17)40-28(35,36)26(30)31/h3-9,11,13-14,22,25-26,38H,2,10,12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50276280

(3-(5-(4-chloro-3-ethylphenoxy)-2 -(3-(1,1,2,2-tetr...)Show SMILES CCc1cc(Oc2cccc3N(CC(O)C(F)(F)F)C(CCc23)c2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C28H25ClF7NO3/c1-2-16-13-18(9-11-21(16)29)39-24-8-4-7-23-20(24)10-12-22(37(23)15-25(38)27(32,33)34)17-5-3-6-19(14-17)40-28(35,36)26(30)31/h3-9,11,13-14,22,25-26,38H,2,10,12,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277811
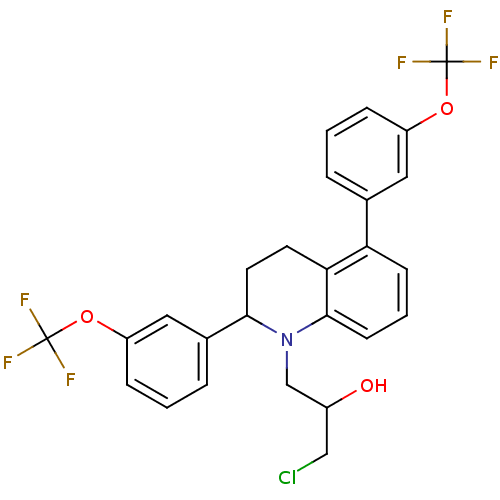
(1-(2,5-bis(3-(trifluoromethoxy)phenyl)-3,4-dihydro...)Show SMILES OC(CCl)CN1C(CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C26H22ClF6NO3/c27-14-18(35)15-34-23(17-5-2-7-20(13-17)37-26(31,32)33)11-10-22-21(8-3-9-24(22)34)16-4-1-6-19(12-16)36-25(28,29)30/h1-9,12-13,18,23,35H,10-11,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 744 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277492
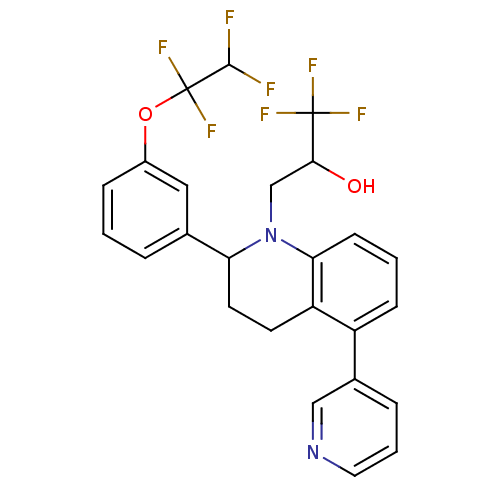
(1,1,1-trifluoro-3-(5-(pyridin-3-yl)-2-(3-(1,1,2,2-...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccnc1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C25H21F7N2O2/c26-23(27)25(31,32)36-17-6-1-4-15(12-17)20-10-9-19-18(16-5-3-11-33-13-16)7-2-8-21(19)34(20)14-22(35)24(28,29)30/h1-8,11-13,20,22-23,35H,9-10,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277886
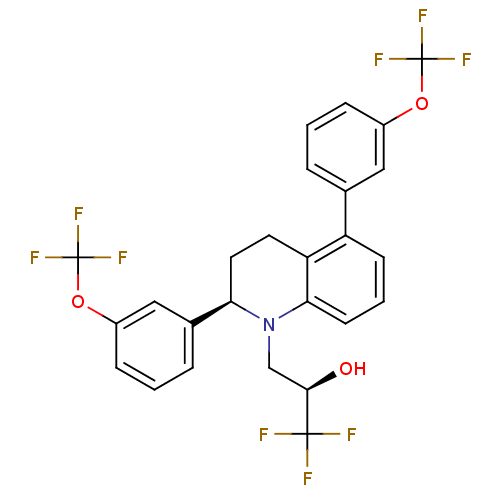
(CHEMBL491072 | Tetrahydroquinoline B)Show SMILES O[C@H](CN1[C@H](CCc2c1cccc2-c1cccc(OC(F)(F)F)c1)c1cccc(OC(F)(F)F)c1)C(F)(F)F |r| Show InChI InChI=1S/C26H20F9NO3/c27-24(28,29)23(37)14-36-21(16-5-2-7-18(13-16)39-26(33,34)35)11-10-20-19(8-3-9-22(20)36)15-4-1-6-17(12-15)38-25(30,31)32/h1-9,12-13,21,23,37H,10-11,14H2/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277557
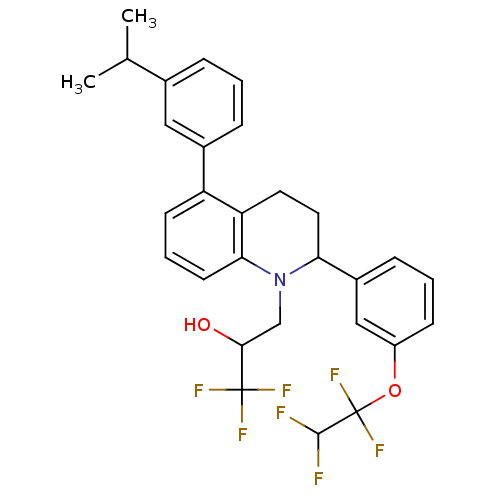
(1,1,1-trifluoro-3-(5-(3-isopropylphenyl)-2-(3-(1,1...)Show SMILES CC(C)c1cccc(c1)-c1cccc2N(CC(O)C(F)(F)F)C(CCc12)c1cccc(OC(F)(F)C(F)F)c1 Show InChI InChI=1S/C29H28F7NO2/c1-17(2)18-6-3-7-19(14-18)22-10-5-11-25-23(22)12-13-24(37(25)16-26(38)28(32,33)34)20-8-4-9-21(15-20)39-29(35,36)27(30)31/h3-11,14-15,17,24,26-27,38H,12-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277556
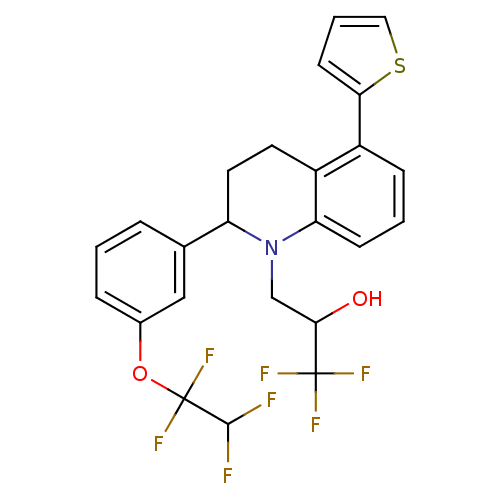
(1,1,1-trifluoro-3-(2-(3-(1,1,2,2-tetrafluoroethoxy...)Show SMILES OC(CN1C(CCc2c1cccc2-c1cccs1)c1cccc(OC(F)(F)C(F)F)c1)C(F)(F)F Show InChI InChI=1S/C24H20F7NO2S/c25-22(26)24(30,31)34-15-5-1-4-14(12-15)18-10-9-16-17(20-8-3-11-35-20)6-2-7-19(16)32(18)13-21(33)23(27,28)29/h1-8,11-12,18,21-22,33H,9-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50277558
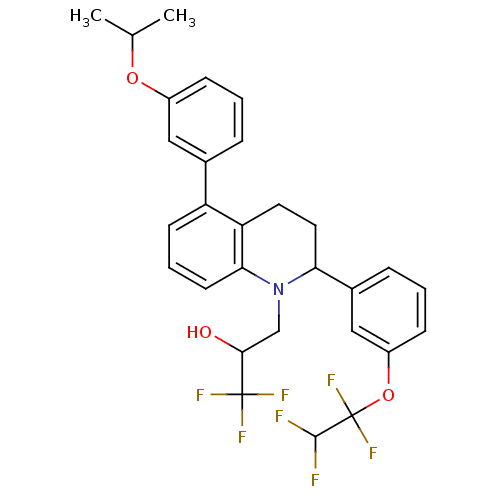
(1,1,1-trifluoro-3-(5-(3-isopropoxyphenyl)-2-(3-(1,...)Show SMILES CC(C)Oc1cccc(c1)-c1cccc2N(CC(O)C(F)(F)F)C(CCc12)c1cccc(OC(F)(F)C(F)F)c1 Show InChI InChI=1S/C29H28F7NO3/c1-17(2)39-20-8-3-6-18(14-20)22-10-5-11-25-23(22)12-13-24(37(25)16-26(38)28(32,33)34)19-7-4-9-21(15-19)40-29(35,36)27(30)31/h3-11,14-15,17,24,26-27,38H,12-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasma derived CETP |
Bioorg Med Chem Lett 19: 2456-60 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.051
BindingDB Entry DOI: 10.7270/Q2W37W6T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data