

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK2 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277568 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277571 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277569 ((4R,5S)-2-Acetyl-5-{(1R)-1-[3,5-bis(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277572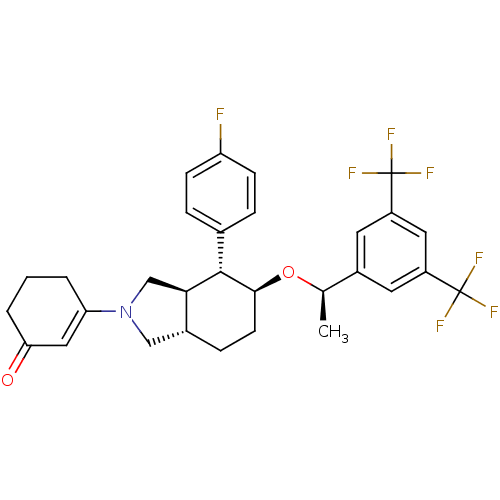 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277510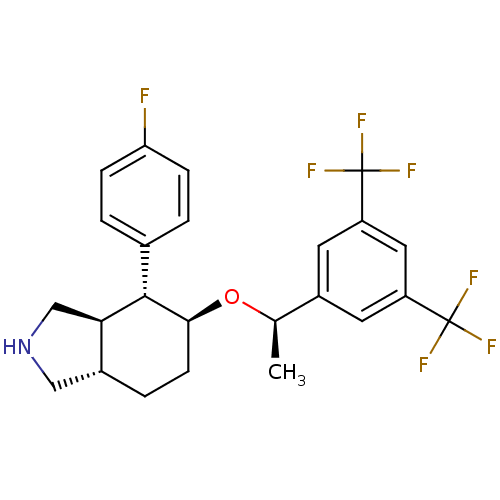 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277567 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277570 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in absence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277510 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277569 ((4R,5S)-2-Acetyl-5-{(1R)-1-[3,5-bis(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277568 ((3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277571 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277567 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277572 (3-[(4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50277570 ((4R,5S)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]substance P human recombinant NK1 receptor expressed in CHO cells in presence of human serum albumin | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP2C8 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50277511 (3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 | J Med Chem 52: 3039-46 (2009) Article DOI: 10.1021/jm8016514 BindingDB Entry DOI: 10.7270/Q2FX79BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||