

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353684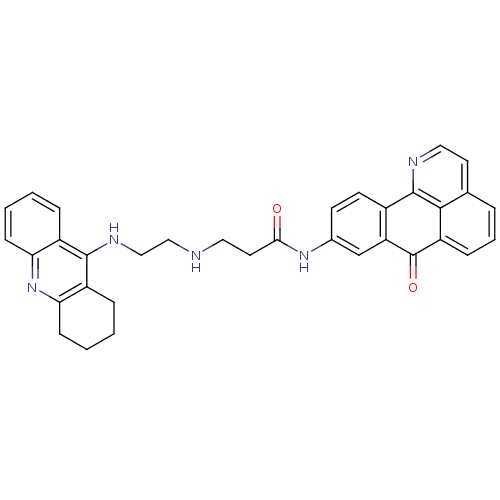 (CHEMBL1830627) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353680 (CHEMBL1830632) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353685 (CHEMBL1830626) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353681 (CHEMBL1830631) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353683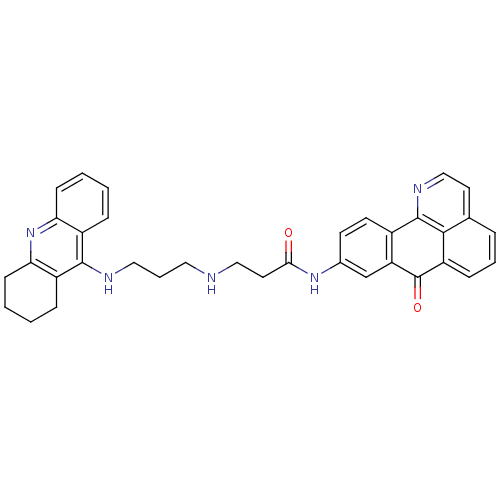 (CHEMBL1830628) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353689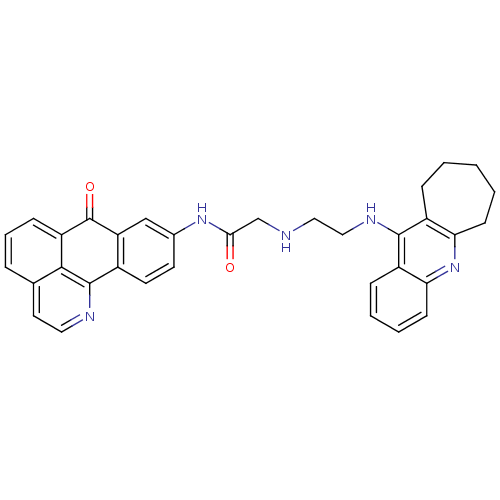 (CHEMBL1830630) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353681 (CHEMBL1830631) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353684 (CHEMBL1830627) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353679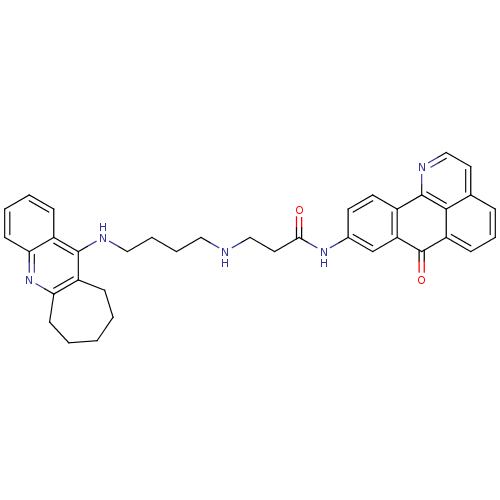 (CHEMBL1830633) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353682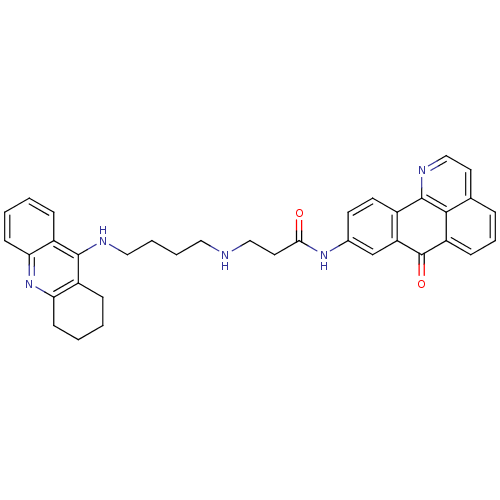 (CHEMBL1830629) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353683 (CHEMBL1830628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353686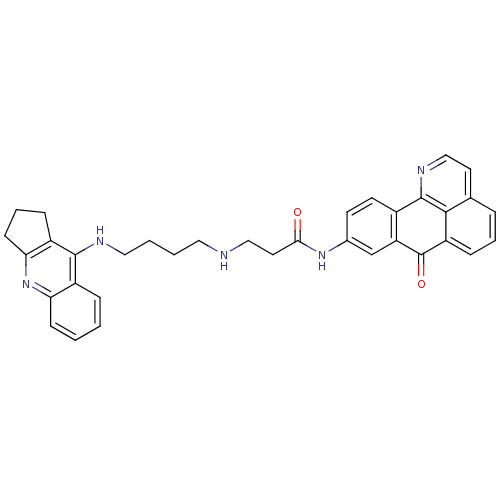 (CHEMBL1830625) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353689 (CHEMBL1830630) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353688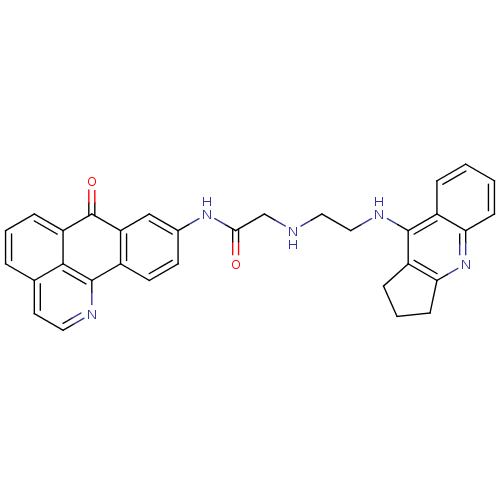 (CHEMBL1830622) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353680 (CHEMBL1830632) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353682 (CHEMBL1830629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353687 (CHEMBL1830624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353687 (CHEMBL1830624) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353690 (CHEMBL1830623) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353685 (CHEMBL1830626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353688 (CHEMBL1830622) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353679 (CHEMBL1830633) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 745 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50353686 (CHEMBL1830625) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50353690 (CHEMBL1830623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50211247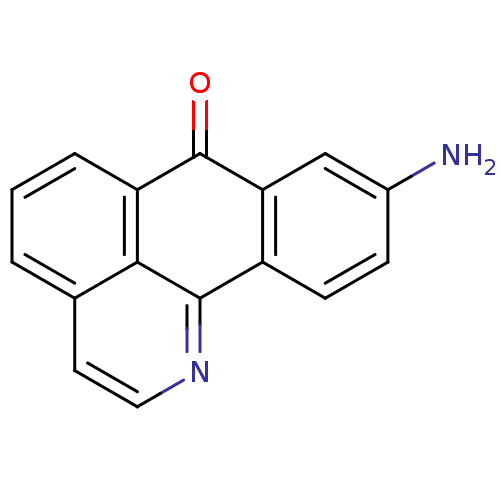 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50211247 (9-Amino-1-azabenzanthrone | 9-amino-1-aza-benzo[de...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using acetylthiocholine chloride as substrate after 15 mins by Ellman's method | Eur J Med Chem 46: 4970-9 (2011) Article DOI: 10.1016/j.ejmech.2011.08.002 BindingDB Entry DOI: 10.7270/Q20C4W5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||