 Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50033968
Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50033968 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM50342149
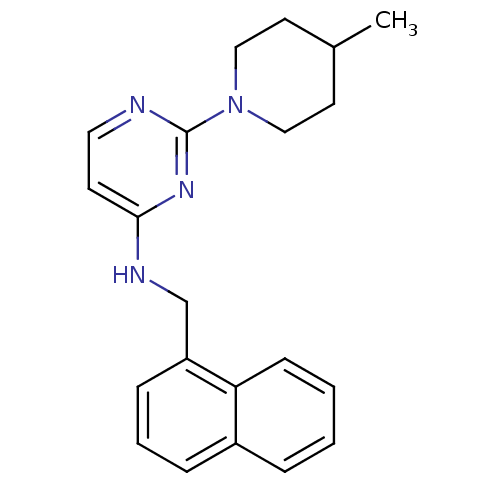
(2-(4-Methylpiperidin-1-yl)-N-(naphth-1-ylmethyl)py...)Show InChI InChI=1S/C21H24N4/c1-16-10-13-25(14-11-16)21-22-12-9-20(24-21)23-15-18-7-4-6-17-5-2-3-8-19(17)18/h2-9,12,16H,10-11,13-15H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50319981
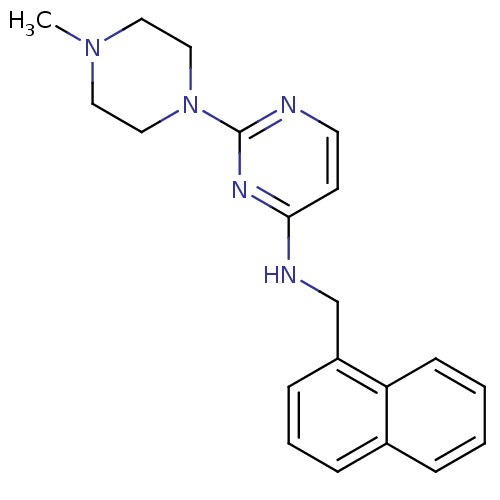
(2-(4-methylpiperazin-1-yl)-N-(naphthalen-1-ylmethy...)Show InChI InChI=1S/C20H23N5/c1-24-11-13-25(14-12-24)20-21-10-9-19(23-20)22-15-17-7-4-6-16-5-2-3-8-18(16)17/h2-10H,11-15H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354727
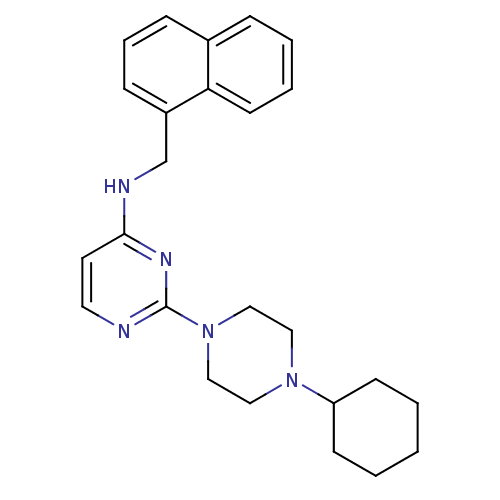
(CHEMBL1834063)Show SMILES C(Nc1ccnc(n1)N1CCN(CC1)C1CCCCC1)c1cccc2ccccc12 Show InChI InChI=1S/C25H31N5/c1-2-10-22(11-3-1)29-15-17-30(18-16-29)25-26-14-13-24(28-25)27-19-21-9-6-8-20-7-4-5-12-23(20)21/h4-9,12-14,22H,1-3,10-11,15-19H2,(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354737
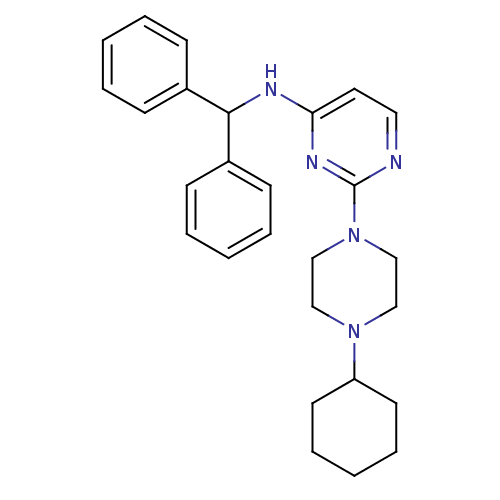
(CHEMBL1834074)Show SMILES C1CCC(CC1)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C27H33N5/c1-4-10-22(11-5-1)26(23-12-6-2-7-13-23)29-25-16-17-28-27(30-25)32-20-18-31(19-21-32)24-14-8-3-9-15-24/h1-2,4-7,10-13,16-17,24,26H,3,8-9,14-15,18-21H2,(H,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354724
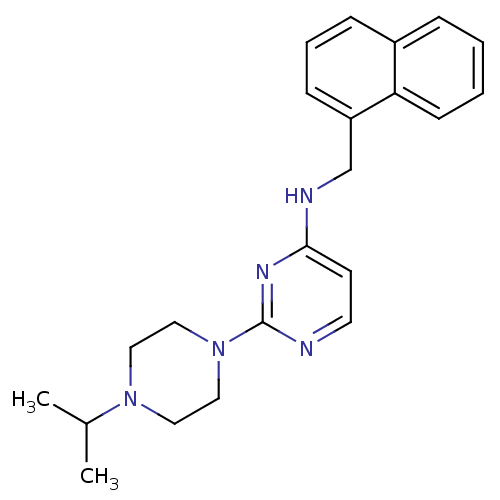
(CHEMBL1834060)Show InChI InChI=1S/C22H27N5/c1-17(2)26-12-14-27(15-13-26)22-23-11-10-21(25-22)24-16-19-8-5-7-18-6-3-4-9-20(18)19/h3-11,17H,12-16H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354727

(CHEMBL1834063)Show SMILES C(Nc1ccnc(n1)N1CCN(CC1)C1CCCCC1)c1cccc2ccccc12 Show InChI InChI=1S/C25H31N5/c1-2-10-22(11-3-1)29-15-17-30(18-16-29)25-26-14-13-24(28-25)27-19-21-9-6-8-20-7-4-5-12-23(20)21/h4-9,12-14,22H,1-3,10-11,15-19H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354725
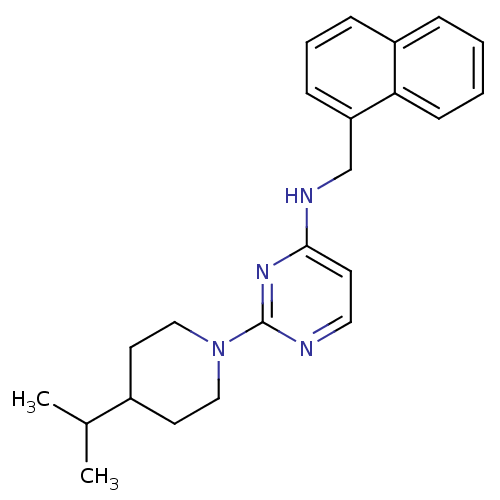
(CHEMBL1834061)Show InChI InChI=1S/C23H28N4/c1-17(2)18-11-14-27(15-12-18)23-24-13-10-22(26-23)25-16-20-8-5-7-19-6-3-4-9-21(19)20/h3-10,13,17-18H,11-12,14-16H2,1-2H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354734
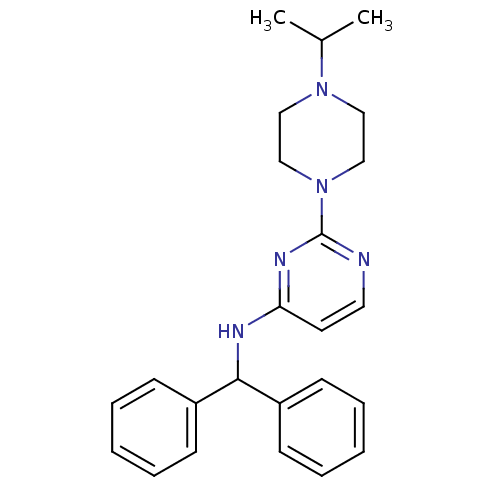
(CHEMBL1834071)Show SMILES CC(C)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5/c1-19(2)28-15-17-29(18-16-28)24-25-14-13-22(27-24)26-23(20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-14,19,23H,15-18H2,1-2H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354730
(CHEMBL1834067)Show InChI InChI=1S/C21H25N5O/c27-15-14-25-10-12-26(13-11-25)21-22-9-8-20(24-21)23-16-18-6-3-5-17-4-1-2-7-19(17)18/h1-9,27H,10-16H2,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354737

(CHEMBL1834074)Show SMILES C1CCC(CC1)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C27H33N5/c1-4-10-22(11-5-1)26(23-12-6-2-7-13-23)29-25-16-17-28-27(30-25)32-20-18-31(19-21-32)24-14-8-3-9-15-24/h1-2,4-7,10-13,16-17,24,26H,3,8-9,14-15,18-21H2,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354731
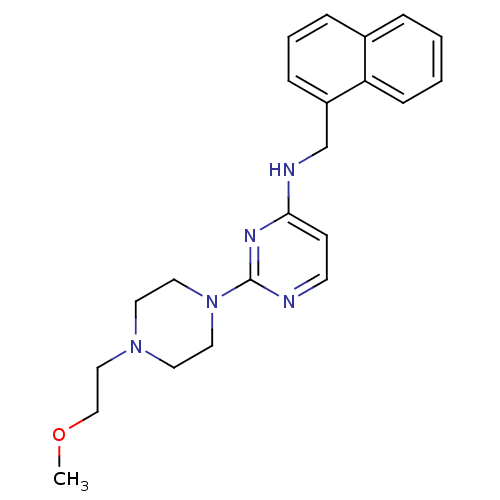
(CHEMBL1834068)Show InChI InChI=1S/C22H27N5O/c1-28-16-15-26-11-13-27(14-12-26)22-23-10-9-21(25-22)24-17-19-7-4-6-18-5-2-3-8-20(18)19/h2-10H,11-17H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354732
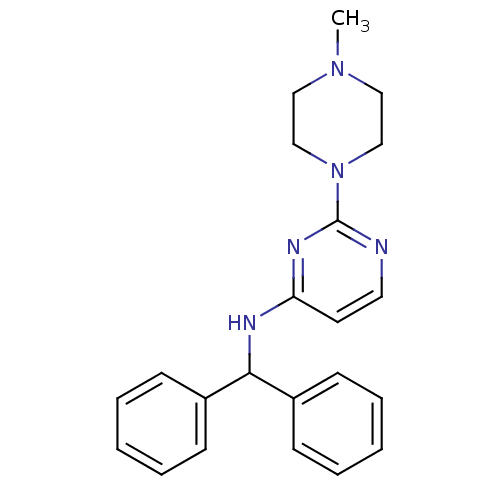
(CHEMBL1834069)Show InChI InChI=1S/C22H25N5/c1-26-14-16-27(17-15-26)22-23-13-12-20(25-22)24-21(18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-13,21H,14-17H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354728
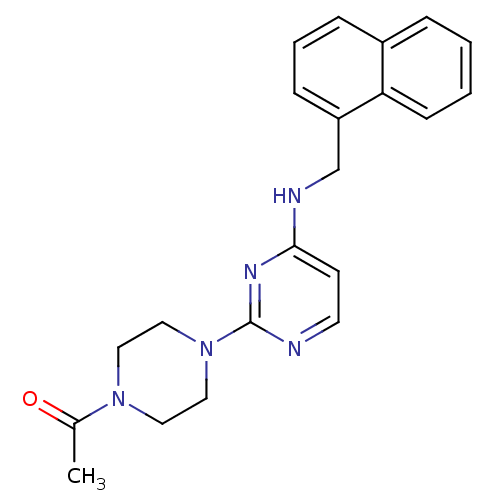
(CHEMBL1834064)Show InChI InChI=1S/C21H23N5O/c1-16(27)25-11-13-26(14-12-25)21-22-10-9-20(24-21)23-15-18-7-4-6-17-5-2-3-8-19(17)18/h2-10H,11-15H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354736
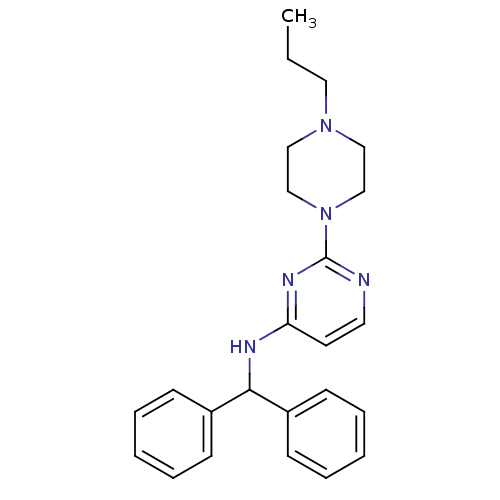
(CHEMBL1834073)Show SMILES CCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5/c1-2-15-28-16-18-29(19-17-28)24-25-14-13-22(27-24)26-23(20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-14,23H,2,15-19H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354724

(CHEMBL1834060)Show InChI InChI=1S/C22H27N5/c1-17(2)26-12-14-27(15-13-26)22-23-11-10-21(25-22)24-16-19-8-5-7-18-6-3-4-9-20(18)19/h3-11,17H,12-16H2,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354725

(CHEMBL1834061)Show InChI InChI=1S/C23H28N4/c1-17(2)18-11-14-27(15-12-18)23-24-13-10-22(26-23)25-16-20-8-5-7-19-6-3-4-9-21(19)20/h3-10,13,17-18H,11-12,14-16H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50319981

(2-(4-methylpiperazin-1-yl)-N-(naphthalen-1-ylmethy...)Show InChI InChI=1S/C20H23N5/c1-24-11-13-25(14-12-24)20-21-10-9-19(23-20)22-15-17-7-4-6-16-5-2-3-8-18(16)17/h2-10H,11-15H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354729
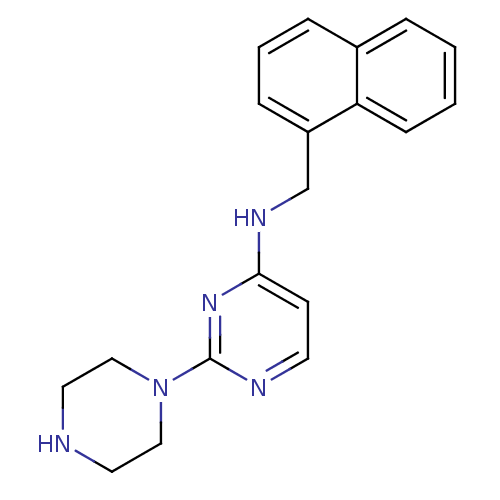
(CHEMBL1834066)Show InChI InChI=1S/C19H21N5/c1-2-7-17-15(4-1)5-3-6-16(17)14-22-18-8-9-21-19(23-18)24-12-10-20-11-13-24/h1-9,20H,10-14H2,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354736

(CHEMBL1834073)Show SMILES CCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5/c1-2-15-28-16-18-29(19-17-28)24-25-14-13-22(27-24)26-23(20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-14,23H,2,15-19H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354730

(CHEMBL1834067)Show InChI InChI=1S/C21H25N5O/c27-15-14-25-10-12-26(13-11-25)21-22-9-8-20(24-21)23-16-18-6-3-5-17-4-1-2-7-19(17)18/h1-9,27H,10-16H2,(H,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354726
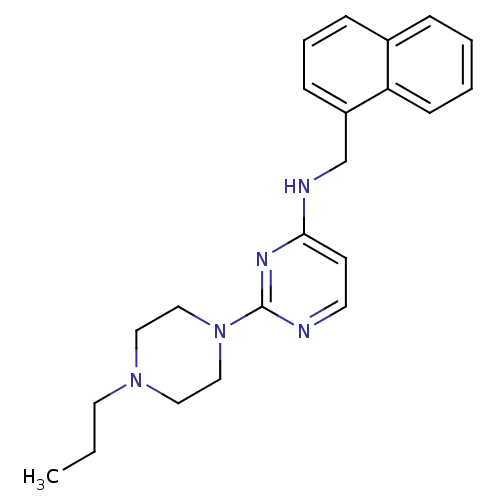
(CHEMBL1834062)Show InChI InChI=1S/C22H27N5/c1-2-12-26-13-15-27(16-14-26)22-23-11-10-21(25-22)24-17-19-8-5-7-18-6-3-4-9-20(18)19/h3-11H,2,12-17H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354726

(CHEMBL1834062)Show InChI InChI=1S/C22H27N5/c1-2-12-26-13-15-27(16-14-26)22-23-11-10-21(25-22)24-17-19-8-5-7-18-6-3-4-9-20(18)19/h3-11H,2,12-17H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354734

(CHEMBL1834071)Show SMILES CC(C)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5/c1-19(2)28-15-17-29(18-16-28)24-25-14-13-22(27-24)26-23(20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-14,19,23H,15-18H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
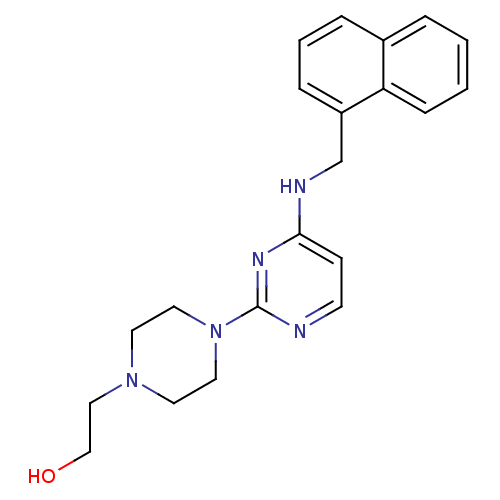
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354739
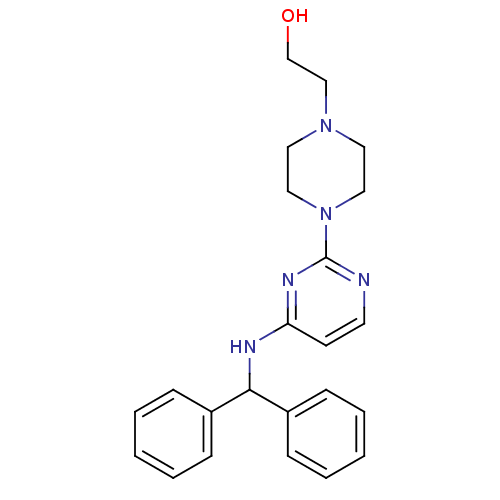
(CHEMBL1834078)Show SMILES OCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C23H27N5O/c29-18-17-27-13-15-28(16-14-27)23-24-12-11-21(26-23)25-22(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-12,22,29H,13-18H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354732

(CHEMBL1834069)Show InChI InChI=1S/C22H25N5/c1-26-14-16-27(17-15-26)22-23-13-12-20(25-22)24-21(18-8-4-2-5-9-18)19-10-6-3-7-11-19/h2-13,21H,14-17H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354729

(CHEMBL1834066)Show InChI InChI=1S/C19H21N5/c1-2-7-17-15(4-1)5-3-6-16(17)14-22-18-8-9-21-19(23-18)24-12-10-20-11-13-24/h1-9,20H,10-14H2,(H,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50342149

(2-(4-Methylpiperidin-1-yl)-N-(naphth-1-ylmethyl)py...)Show InChI InChI=1S/C21H24N4/c1-16-10-13-25(14-11-16)21-22-12-9-20(24-21)23-15-18-7-4-6-17-5-2-3-8-19(17)18/h2-9,12,16H,10-11,13-15H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354731

(CHEMBL1834068)Show InChI InChI=1S/C22H27N5O/c1-28-16-15-26-11-13-27(14-12-26)22-23-10-9-21(25-22)24-17-19-7-4-6-18-5-2-3-8-20(18)19/h2-10H,11-17H2,1H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354723
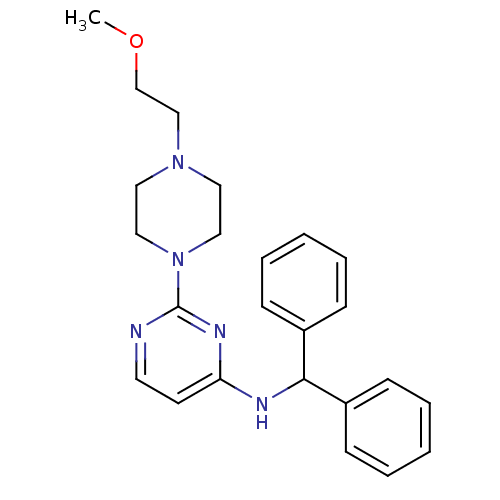
(CHEMBL1834079)Show SMILES COCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5O/c1-30-19-18-28-14-16-29(17-15-28)24-25-13-12-22(27-24)26-23(20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-13,23H,14-19H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354738
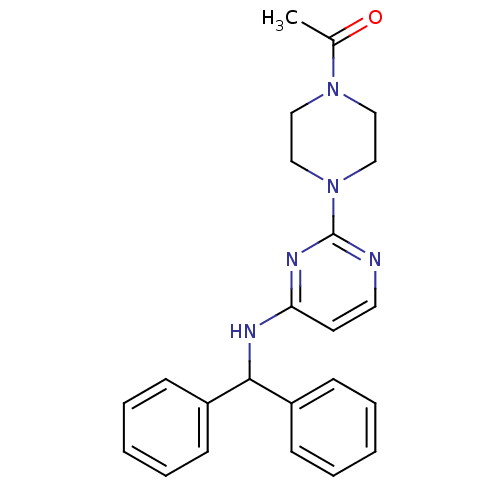
(CHEMBL1834075)Show SMILES CC(=O)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C23H25N5O/c1-18(29)27-14-16-28(17-15-27)23-24-13-12-21(26-23)25-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-13,22H,14-17H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354722
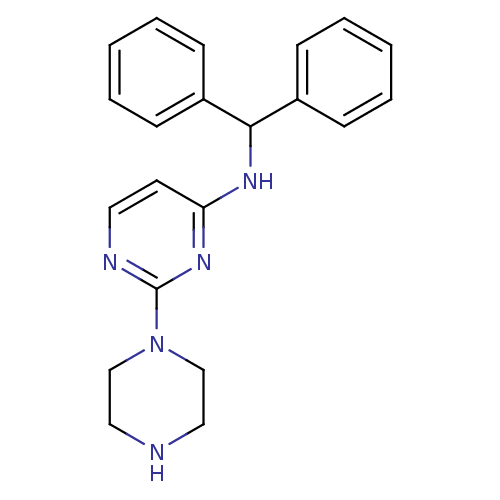
(CHEMBL1834077)Show InChI InChI=1S/C21H23N5/c1-3-7-17(8-4-1)20(18-9-5-2-6-10-18)24-19-11-12-23-21(25-19)26-15-13-22-14-16-26/h1-12,20,22H,13-16H2,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
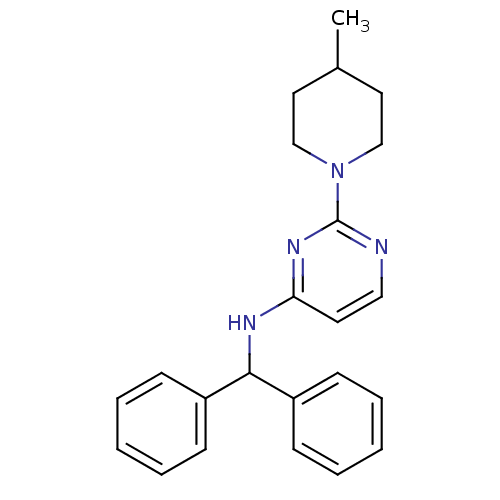
(Homo sapiens (Human)) | BDBM50354733
(CHEMBL1834070)Show InChI InChI=1S/C23H26N4/c1-18-13-16-27(17-14-18)23-24-15-12-21(26-23)25-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-12,15,18,22H,13-14,16-17H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354728

(CHEMBL1834064)Show InChI InChI=1S/C21H23N5O/c1-16(27)25-11-13-26(14-12-25)21-22-10-9-20(24-21)23-15-18-7-4-6-17-5-2-3-8-19(17)18/h2-10H,11-15H2,1H3,(H,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354733

(CHEMBL1834070)Show InChI InChI=1S/C23H26N4/c1-18-13-16-27(17-14-18)23-24-15-12-21(26-23)25-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-12,15,18,22H,13-14,16-17H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354723

(CHEMBL1834079)Show SMILES COCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C24H29N5O/c1-30-19-18-28-14-16-29(17-15-28)24-25-13-12-22(27-24)26-23(20-8-4-2-5-9-20)21-10-6-3-7-11-21/h2-13,23H,14-19H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354735
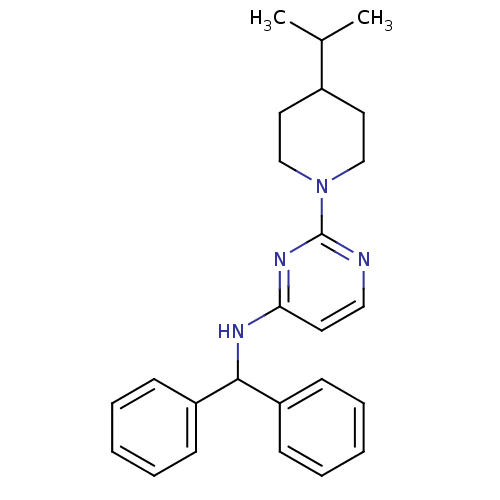
(CHEMBL1834072)Show SMILES CC(C)C1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C25H30N4/c1-19(2)20-14-17-29(18-15-20)25-26-16-13-23(28-25)27-24(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-13,16,19-20,24H,14-15,17-18H2,1-2H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354721

(CHEMBL1834065)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1nccc(NCc2cccc3ccccc23)n1 Show InChI InChI=1S/C24H29N5O2/c1-24(2,3)31-23(30)29-15-13-28(14-16-29)22-25-12-11-21(27-22)26-17-19-9-6-8-18-7-4-5-10-20(18)19/h4-12H,13-17H2,1-3H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354739

(CHEMBL1834078)Show SMILES OCCN1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C23H27N5O/c29-18-17-27-13-15-28(16-14-27)23-24-12-11-21(26-23)25-22(19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-12,22,29H,13-18H2,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354735

(CHEMBL1834072)Show SMILES CC(C)C1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C25H30N4/c1-19(2)20-14-17-29(18-15-20)25-26-16-13-23(28-25)27-24(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-13,16,19-20,24H,14-15,17-18H2,1-2H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354738

(CHEMBL1834075)Show SMILES CC(=O)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C23H25N5O/c1-18(29)27-14-16-28(17-15-27)23-24-13-12-21(26-23)25-22(19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-13,22H,14-17H2,1H3,(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354722

(CHEMBL1834077)Show InChI InChI=1S/C21H23N5/c1-3-7-17(8-4-1)20(18-9-5-2-6-10-18)24-19-11-12-23-21(25-19)26-15-13-22-14-16-26/h1-12,20,22H,13-16H2,(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354720
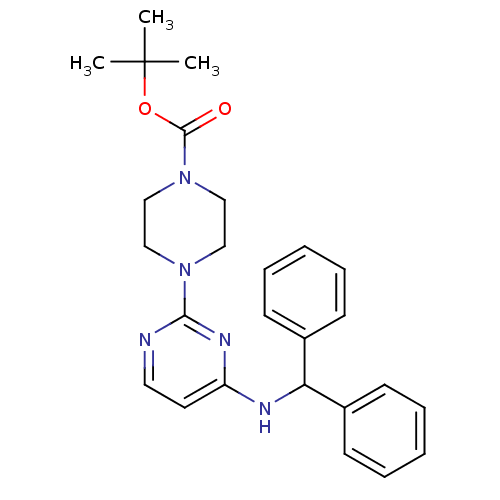
(CHEMBL1834076)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C26H31N5O2/c1-26(2,3)33-25(32)31-18-16-30(17-19-31)24-27-15-14-22(29-24)28-23(20-10-6-4-7-11-20)21-12-8-5-9-13-21/h4-15,23H,16-19H2,1-3H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50354721

(CHEMBL1834065)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1nccc(NCc2cccc3ccccc23)n1 Show InChI InChI=1S/C24H29N5O2/c1-24(2,3)31-23(30)29-15-13-28(14-16-29)22-25-12-11-21(27-22)26-17-19-9-6-8-18-7-4-5-10-20(18)19/h4-12H,13-17H2,1-3H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of equine BuChE by Ellman's method |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50354720

(CHEMBL1834076)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1nccc(NC(c2ccccc2)c2ccccc2)n1 Show InChI InChI=1S/C26H31N5O2/c1-26(2,3)33-25(32)31-18-16-30(17-19-31)24-27-15-14-22(29-24)28-23(20-10-6-4-7-11-20)21-12-8-5-9-13-21/h4-15,23H,16-19H2,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human AChE by Ellman's assay |
Bioorg Med Chem Lett 21: 5881-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.091
BindingDB Entry DOI: 10.7270/Q2C829P7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data