

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464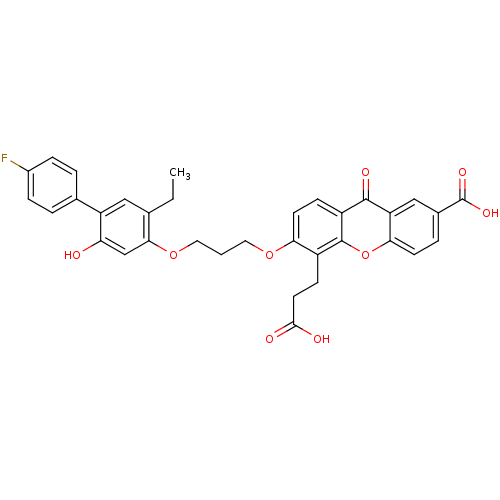 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052027 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for binding affinity against human neutrophil LTB4 (leukotriene) receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052025 (6-{2-(2-Carboxy-ethyl)-3-[6-(4-oxo-8-propyl-chroma...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037382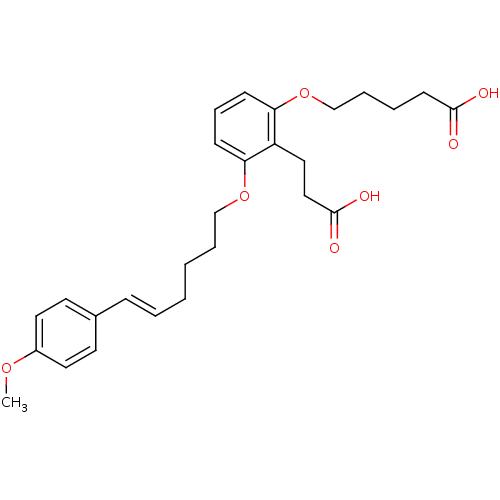 (5-{2-(2-Carboxy-ethyl)-3-[(E)-6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385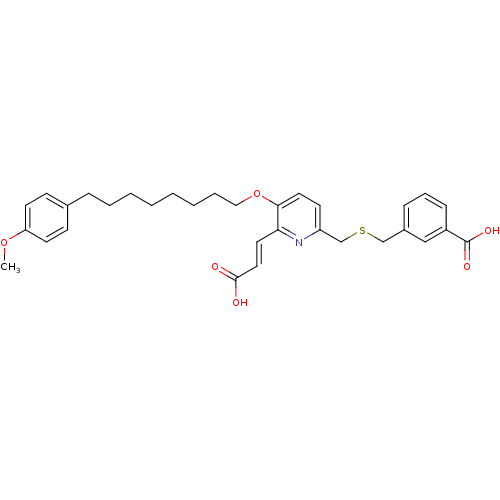 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Leukotriene B4 receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029450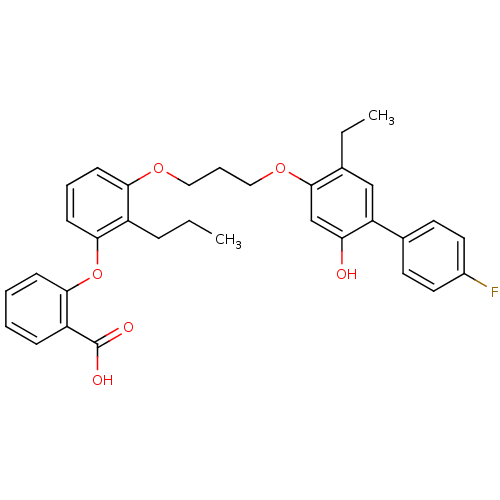 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50052024 (CHEMBL787 | montelukast) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound were tested for inhibitory activity against Cysteinyl leukotriene D4 receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037390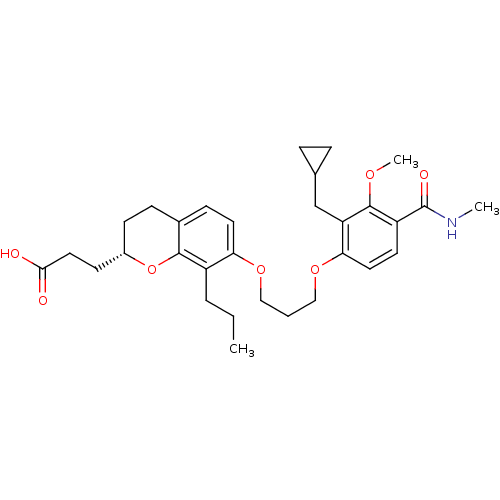 (3-{(S)-2-(2-Carboxy-ethyl)-7-[3-(2-cyclopropylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052017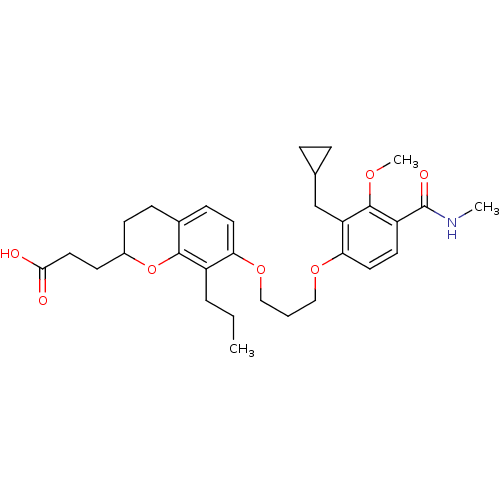 (3-{7-[3-(2-Cyclopropylmethyl-3-methoxy-4-methylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50052029 (CHEMBL89340 | L-668019 | MK-679 | Sodium; 3-[(R)-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound were tested for inhibitory activity against Cysteinyl leukotriene D4 receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052021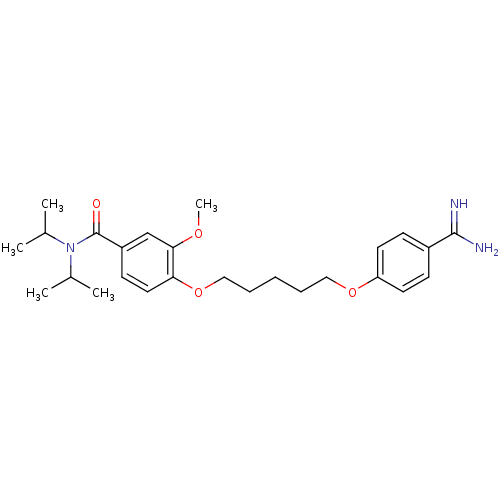 (4-[5-(4-Carbamimidoyl-phenoxy)-pentyloxy]-N,N-diis...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against Leukotriene B4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029482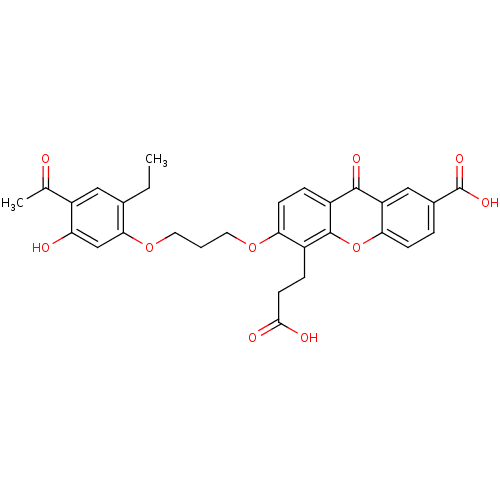 (6-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037218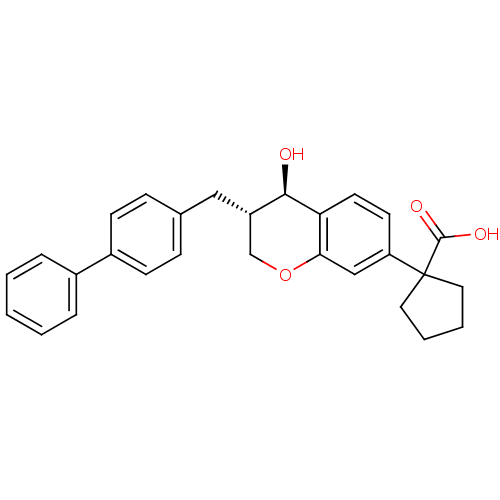 (1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against Leukotriene B4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611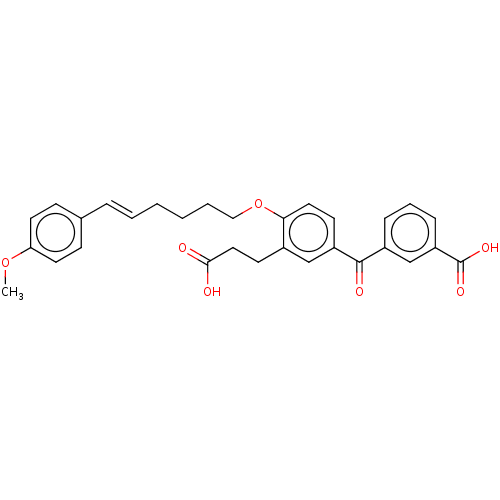 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001610 (7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against LTB4 (leukotriene) binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50006798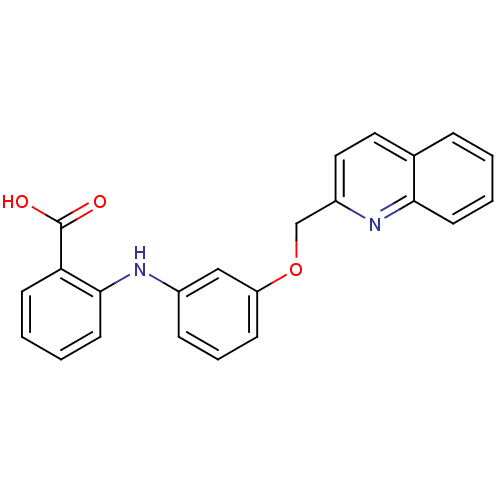 (2-[3-(Quinolin-2-ylmethoxy)-phenylamino]-benzoic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against LTD4 (leukotriene). | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of compound for 5-lipoxygenase activating protein protein by FLAP binding assay | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052028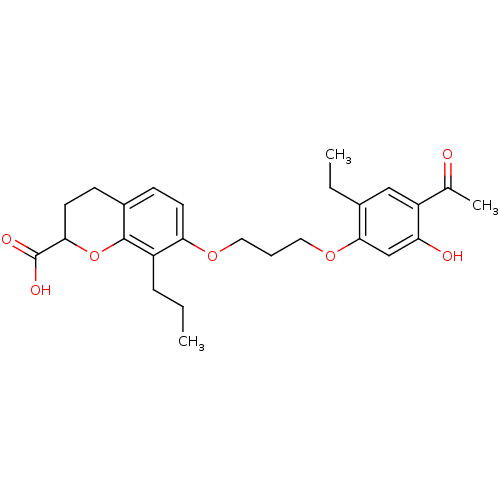 (7-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM81519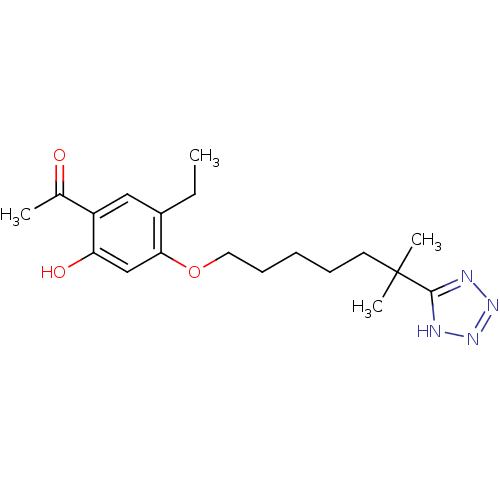 (CAS_117690-79-6 | CGS 23356 | CHEMBL15766 | LY 255...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50009071 (6-Acetyl-7-[5-(4-acetyl-3-hydroxy-2-propyl-phenoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against LTD4 (leukotriene). | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (Homo sapiens (Human)) | BDBM50006812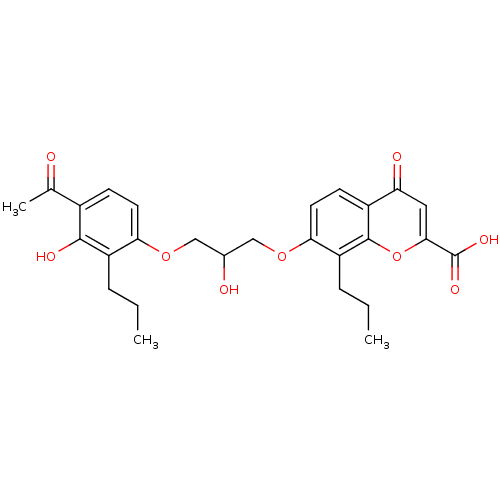 (7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cysteinyl leukotriene D4 receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||