

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50059523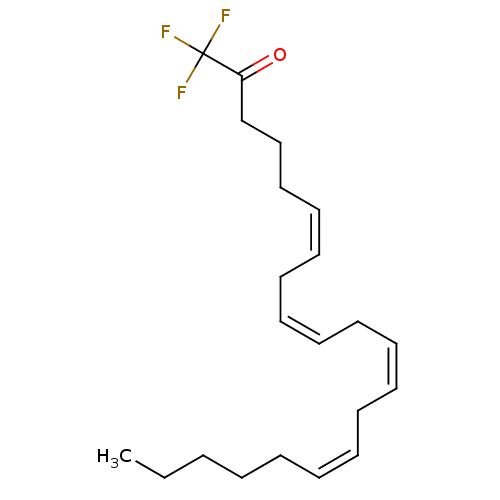 ((6Z,9Z,12Z,15Z)-1,1,1-trifluorohenicosa-6,9,12,15-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414921 (CHEMBL570812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50132713 (Arachidonic acid derivative | CHEMBL113262 | Methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM26736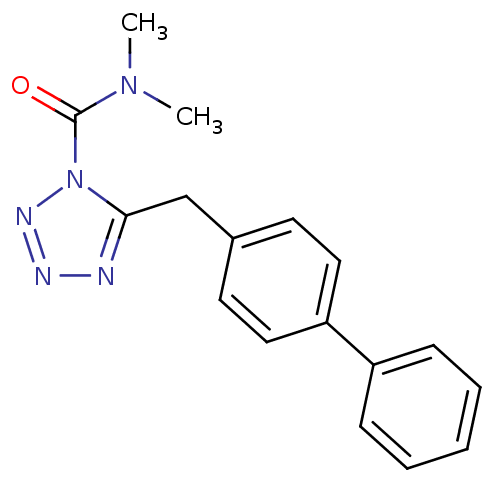 (CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM26736 (CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414921 (CHEMBL570812) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414919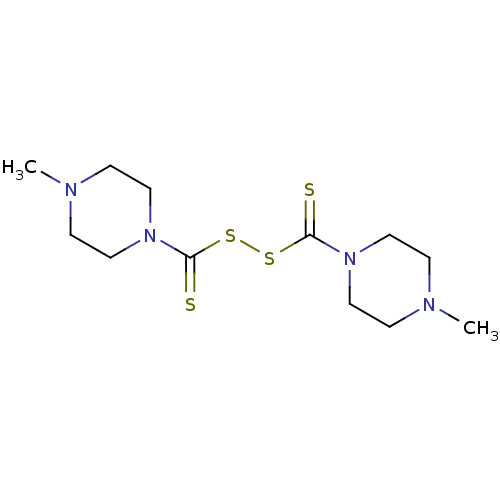 (CHEMBL571699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414940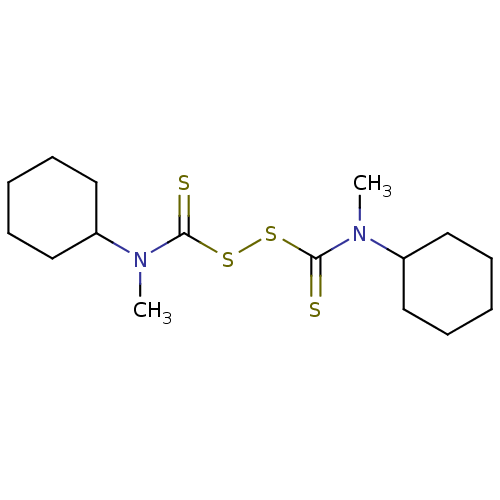 (CHEMBL576697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414953 (CHEMBL570565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414944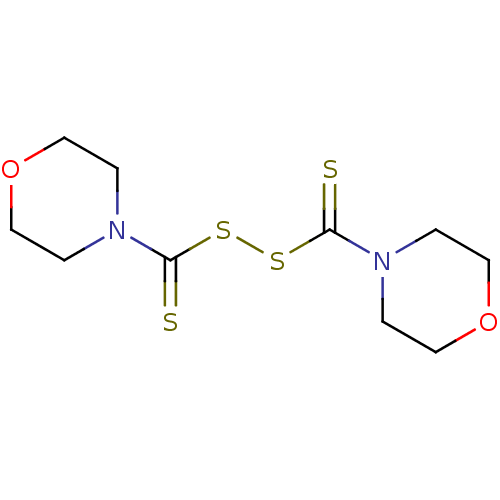 (CHEMBL121556 | NSC-402538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414947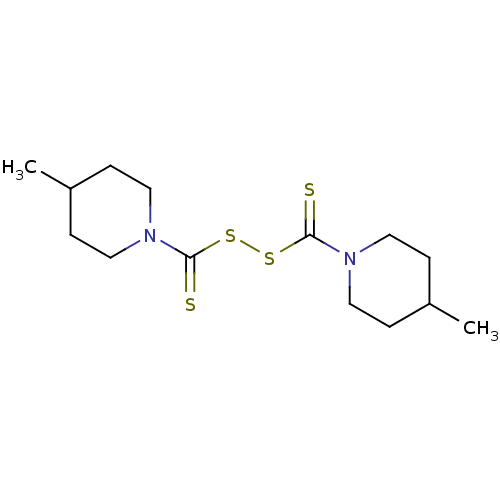 (CHEMBL576671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414943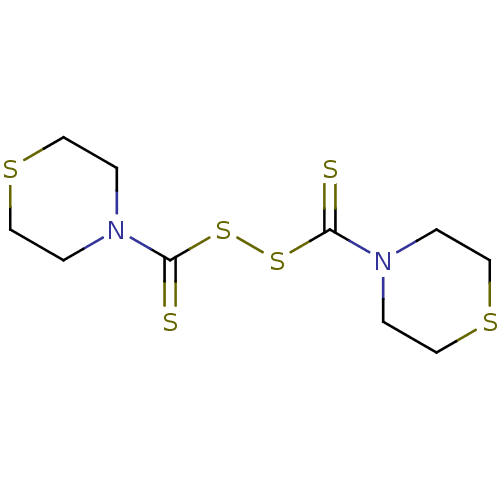 (CHEMBL573920) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414948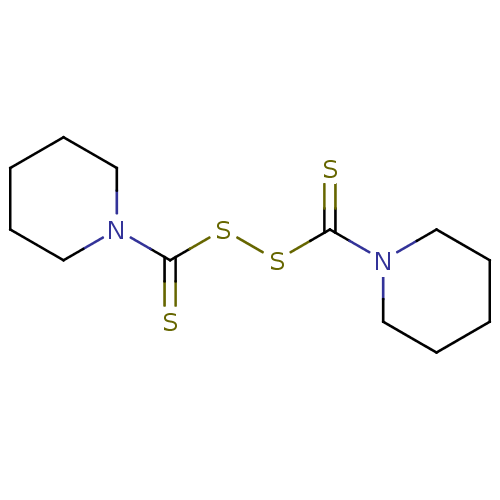 (CHEMBL121516 | NSC-527035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414937 (CHEMBL583524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414938 (CHEMBL571274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 437 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414946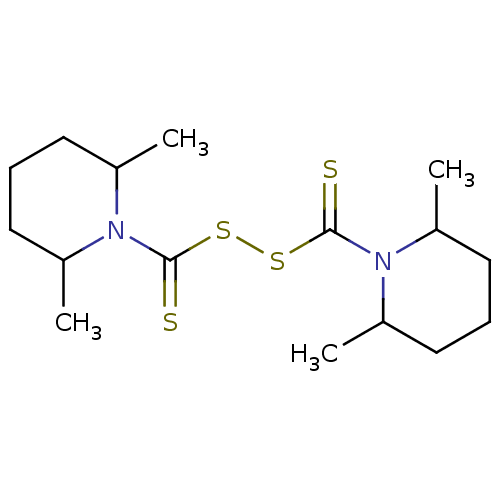 (CHEMBL571273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414942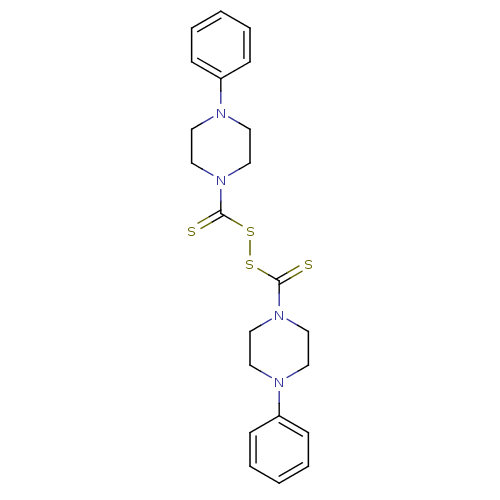 (CHEMBL569626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414939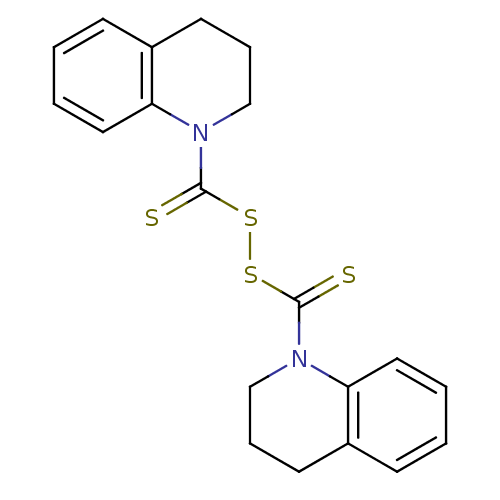 (CHEMBL570340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414952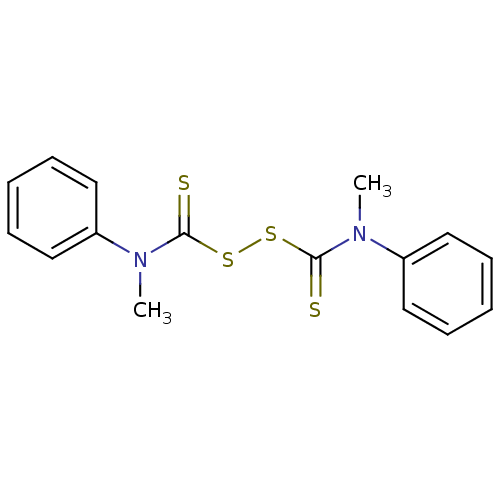 (CHEMBL570804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414950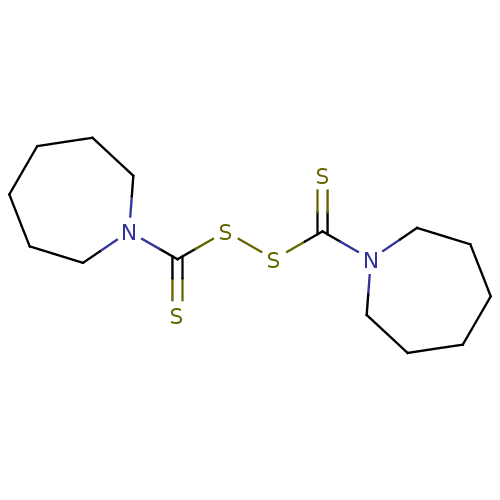 (CHEMBL585179) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 813 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50361476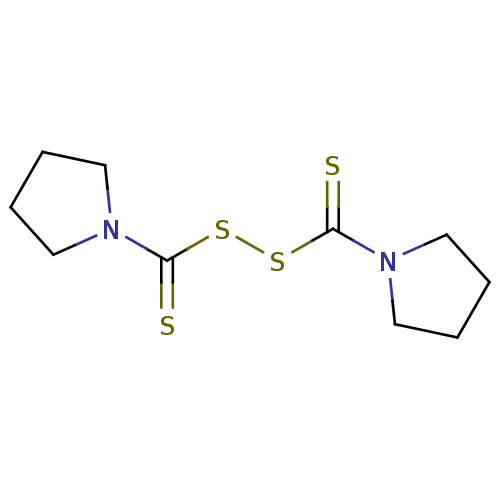 (CHEMBL583959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 912 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM43362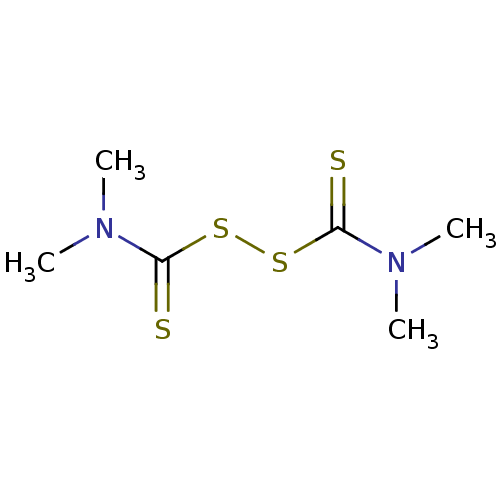 (MLS000069752 | N,N-dimethylcarbamodithioic acid (d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 977 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414920 (CHEMBL571495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50058655 (1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414920 (CHEMBL571495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414951 (CHEMBL578805 | US11753371, Compound II-2h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414940 (CHEMBL576697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414941 (CHEMBL570572) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50128581 (Biphenyl-3-yl-carbamic acid cyclohexyl ester | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414925 (CHEMBL571701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50059523 ((6Z,9Z,12Z,15Z)-1,1,1-trifluorohenicosa-6,9,12,15-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414945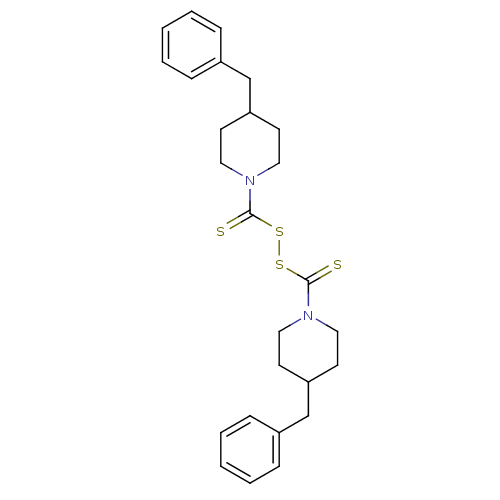 (CHEMBL583000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414922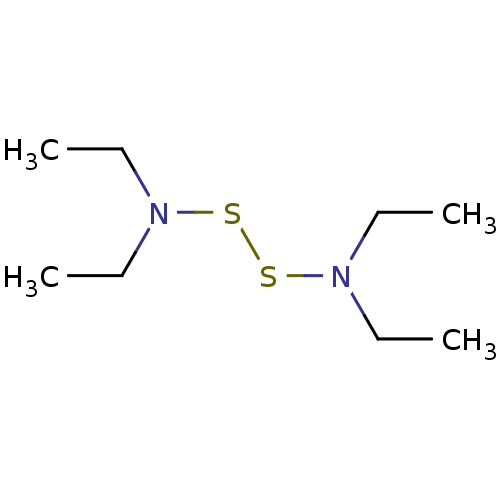 (CHEMBL570577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414923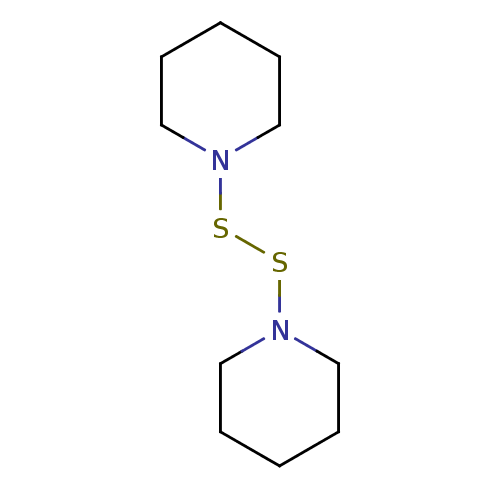 (CHEMBL573921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414936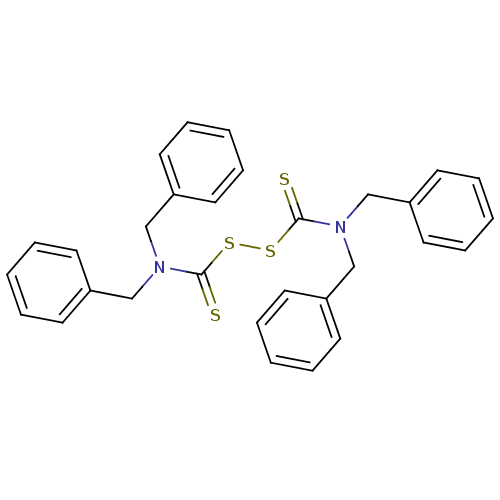 (CHEMBL120082 | NSC-608475 | US11753371, Compound I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50128581 (Biphenyl-3-yl-carbamic acid cyclohexyl ester | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414935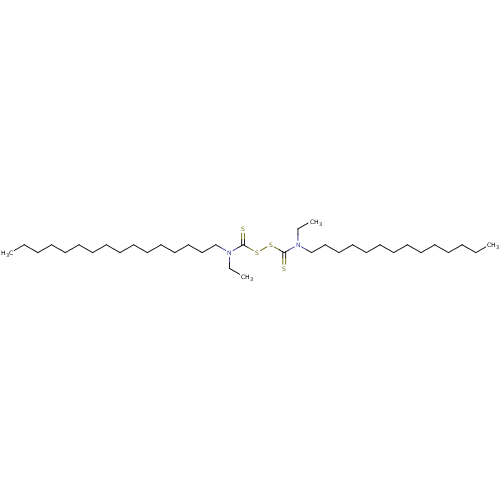 (CHEMBL567397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414924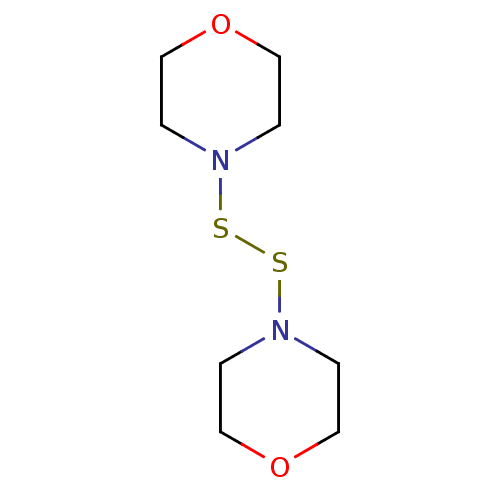 (DITHIODIMORPHOLINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414949 (CHEMBL571700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414926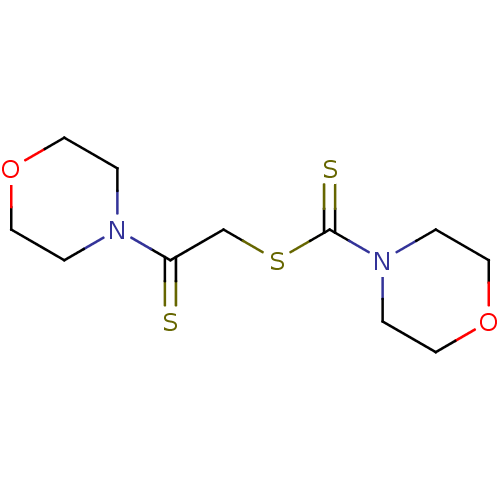 (CHEMBL583322) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Rattus norvegicus) | BDBM50414919 (CHEMBL571699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of recombinant rat ALDH activity in liver mitochondria | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414927 (CHEMBL582931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414934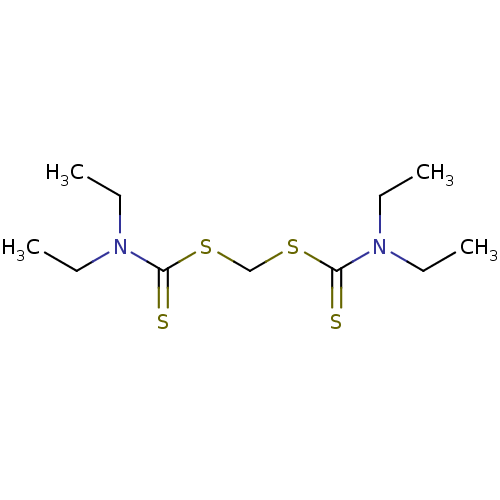 (CHEMBL570813) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414943 (CHEMBL573920) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414952 (CHEMBL570804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde dehydrogenase, mitochondrial (Rattus norvegicus) | BDBM50058655 (1,1',1'',1'''-[disulfanediylbis(carbonothioylnitri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of recombinant rat ALDH activity in liver mitochondria | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM43362 (MLS000069752 | N,N-dimethylcarbamodithioic acid (d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50414944 (CHEMBL121556 | NSC-402538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH-maltose binding protein | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50414932 (CHEMBL570228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of human MGL activity using [3H]2-oleoylglycerol substrate by liquid scintillation counting | J Med Chem 52: 7310-4 (2009) Article DOI: 10.1021/jm901323s BindingDB Entry DOI: 10.7270/Q2319X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |