

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347953 (CHEMBL1800140 | US8637558, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347952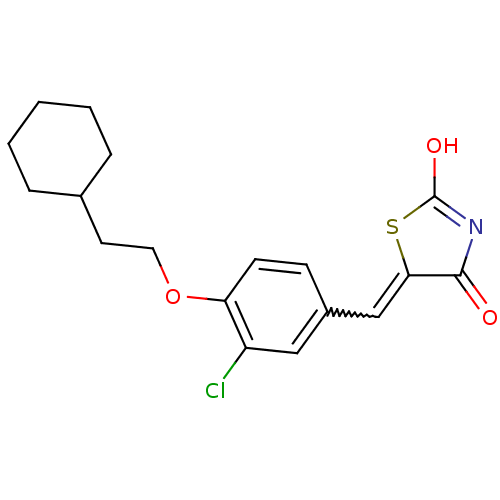 (CHEMBL1800139 | US8637558, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347956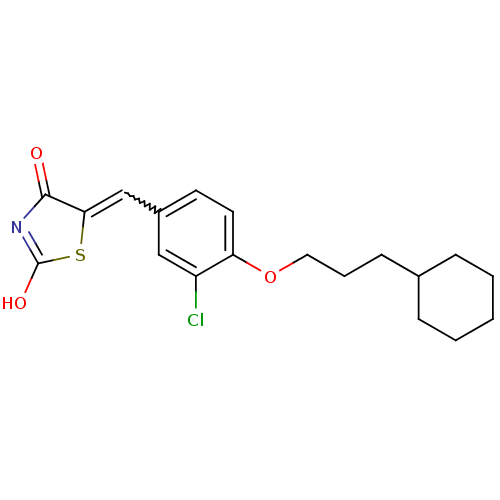 (CHEMBL1800143 | US8637558, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118019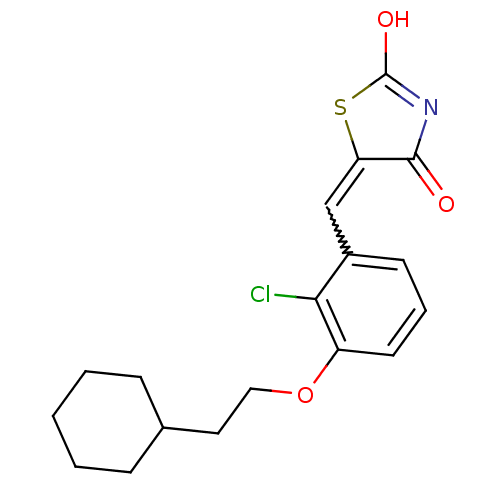 (US8637558, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438430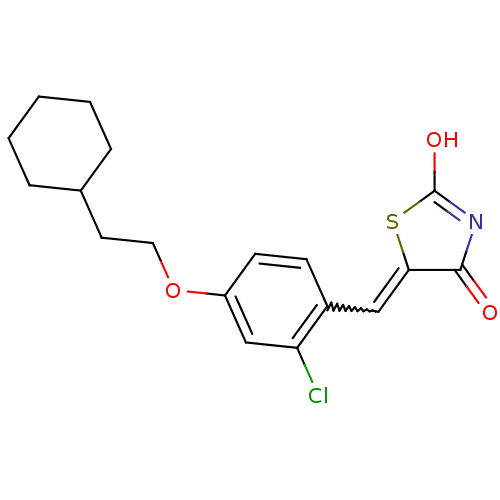 (CHEMBL2414240 | US8637558, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438403 (CHEMBL2414160 | US8637558, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19.8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118042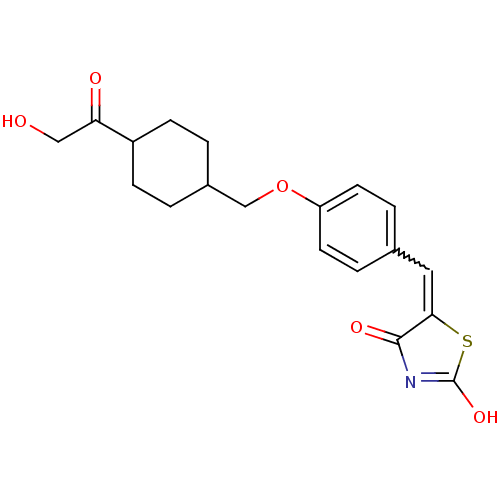 (US8637558, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118056 (US8637558, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22.1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118012 (US8637558, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347957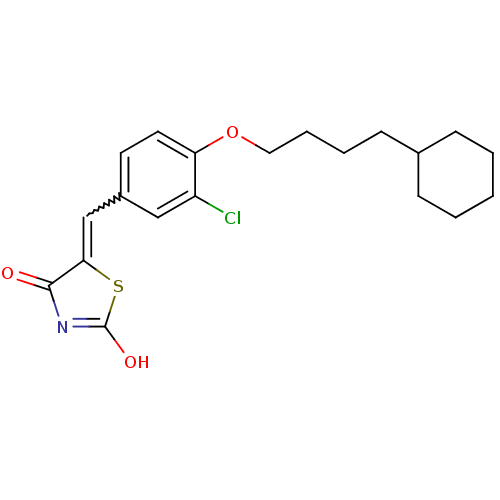 (CHEMBL1800144 | US8637558, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347955 (CHEMBL1800142 | US8637558, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 26.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118057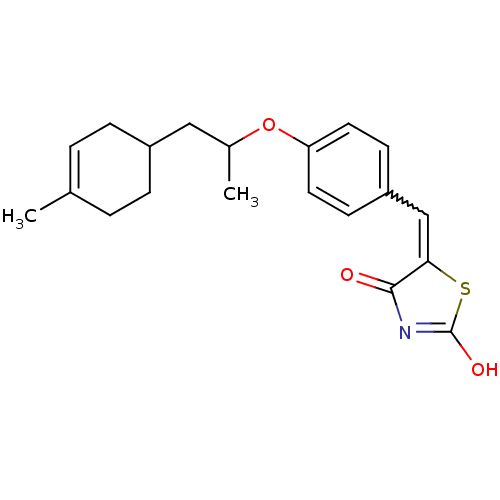 (US8637558, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118041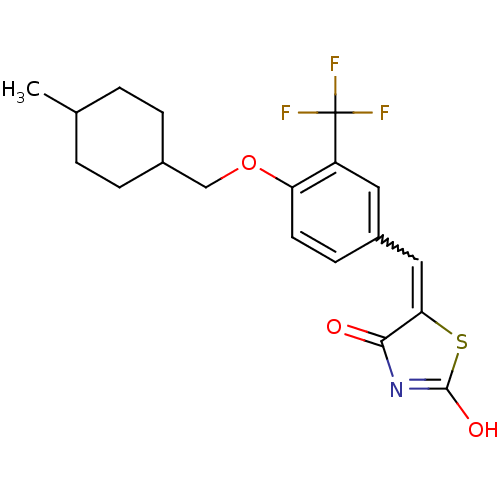 (US8637558, 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438402 (CHEMBL2414161 | US8637558, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 38.1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378637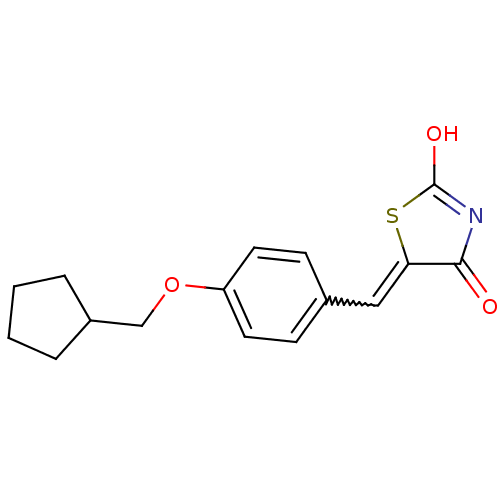 (CHEMBL84603 | US8637558, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118047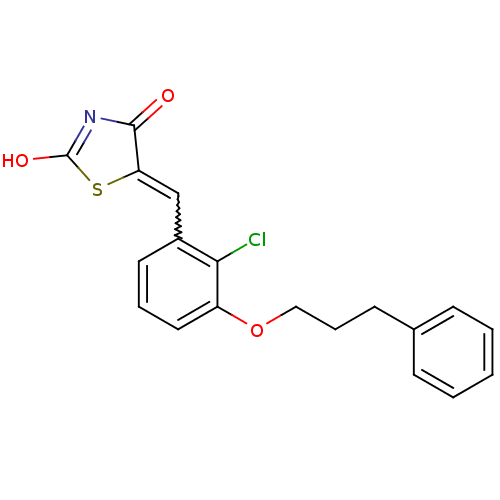 (US8637558, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438404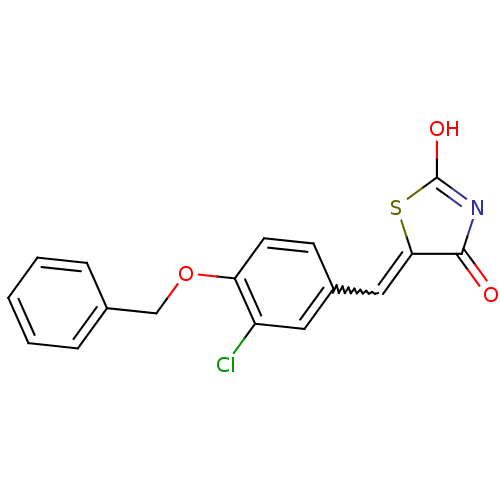 (CHEMBL2414159 | US8637558, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438401 (CHEMBL2414162 | US8637558, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347954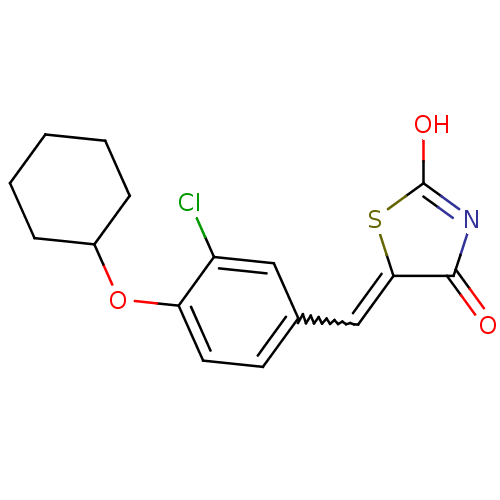 (CHEMBL1800141 | US8637558, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 48.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118029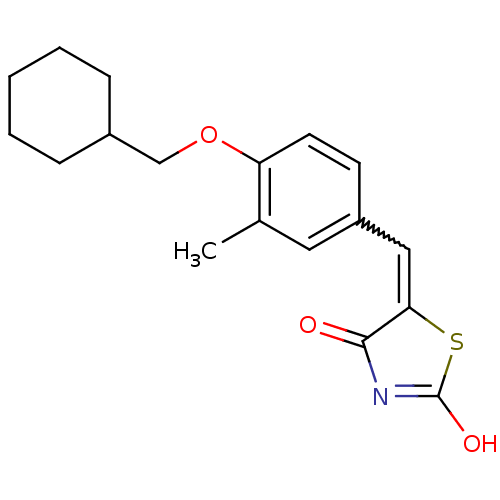 (US8637558, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 49.8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118050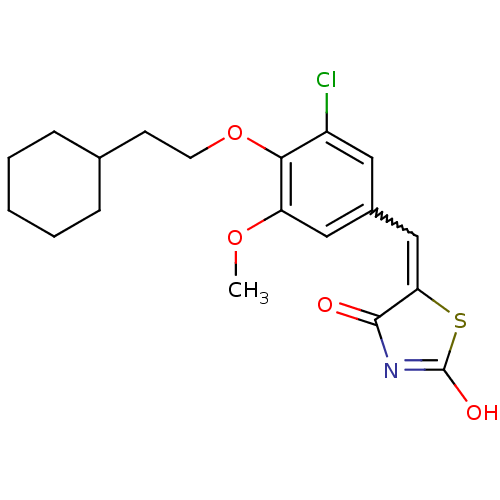 (US8637558, 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118018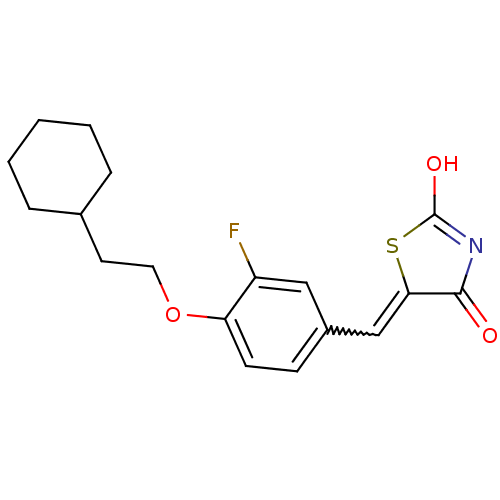 (US8637558, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50.8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50347949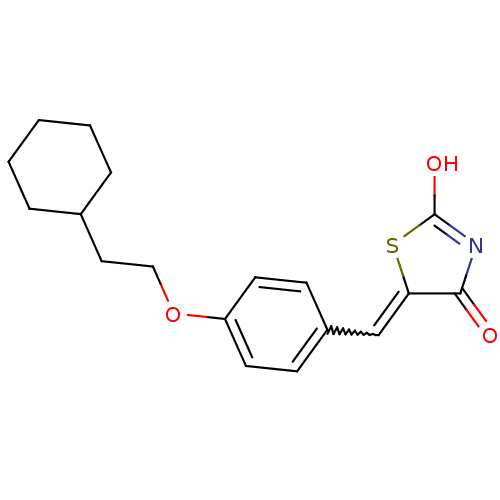 (CHEMBL599406 | US8637558, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 50.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438398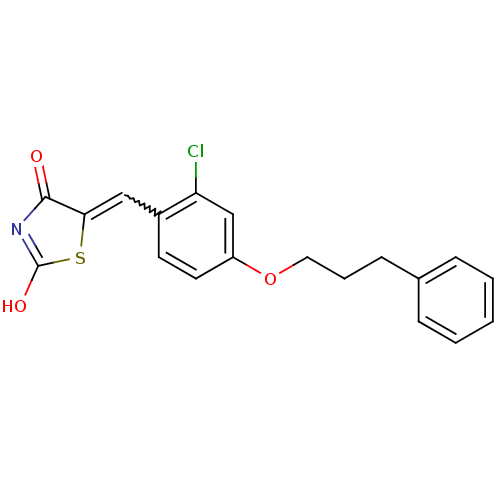 (CHEMBL2414165 | US8637558, 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118046 (US8637558, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118013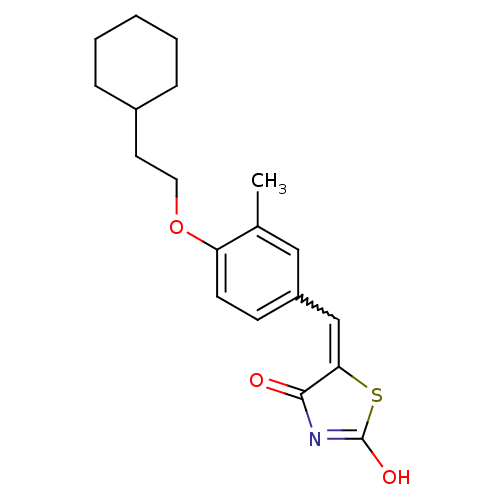 (US8637558, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118039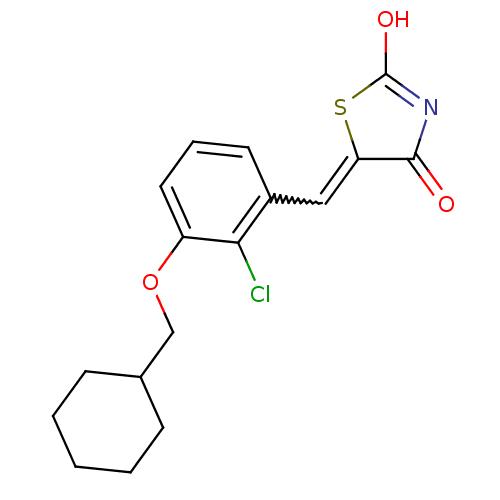 (US8637558, 63) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118040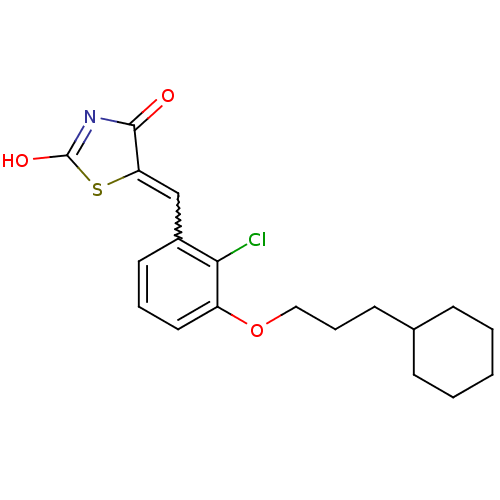 (US8637558, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118027 (US8637558, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 59.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118025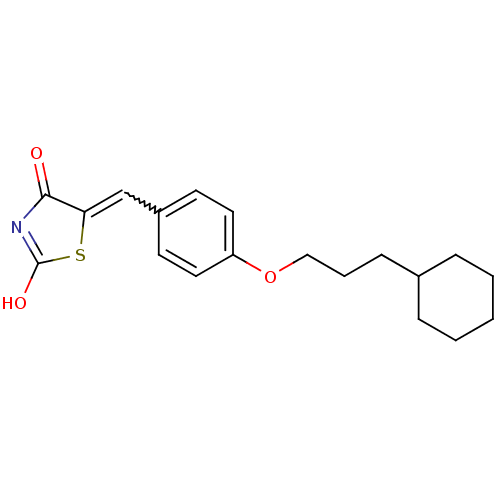 (US8637558, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 62.2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378639 (CHEMBL599018 | US8637558, 37) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 66.4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118017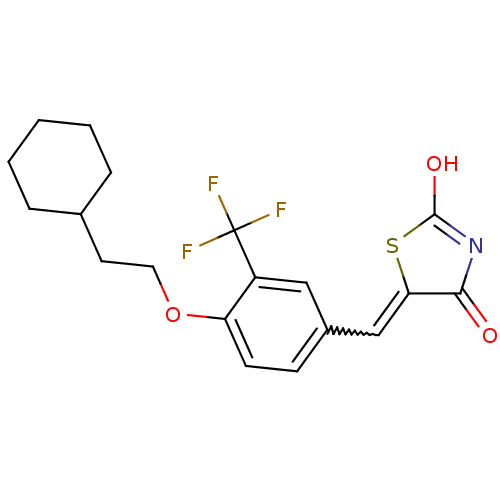 (US8637558, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 71.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438397 (CHEMBL2414166 | US8637558, 88) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50438423 (CHEMBL2414140 | US8637558, 101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 84.1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118067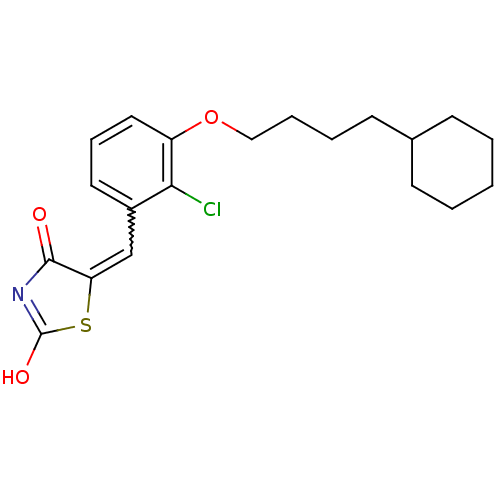 (US8637558, 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 94.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118014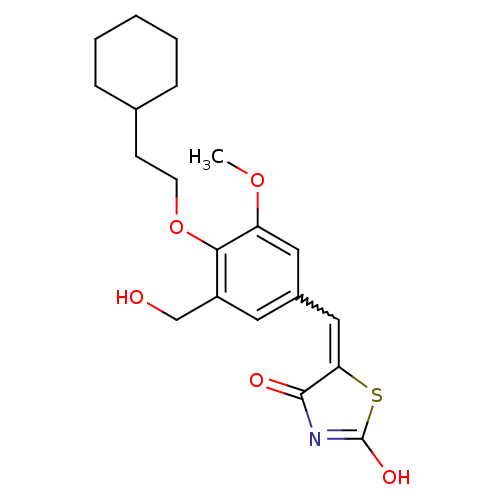 (US8637558, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 94.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378619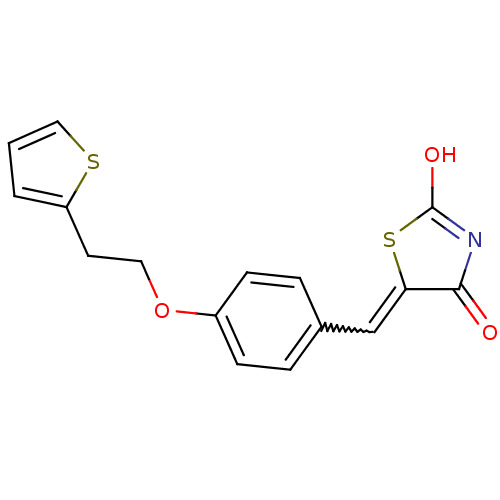 (CHEMBL590265 | US8637558, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95.9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118073 (CHEMBL2414145 | US8637558, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 99.3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118030 (US8637558, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 104 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118052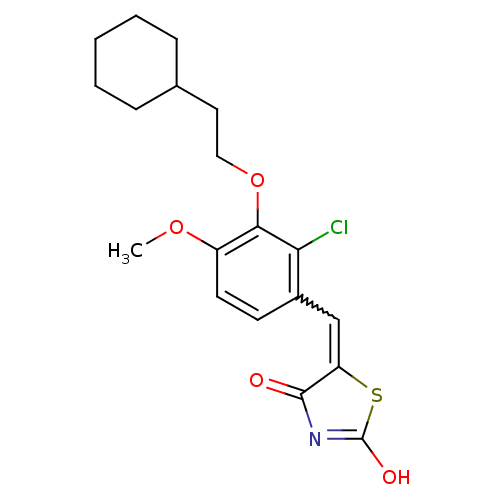 (US8637558, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378631 (CHEMBL598397 | US8637558, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 115 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118033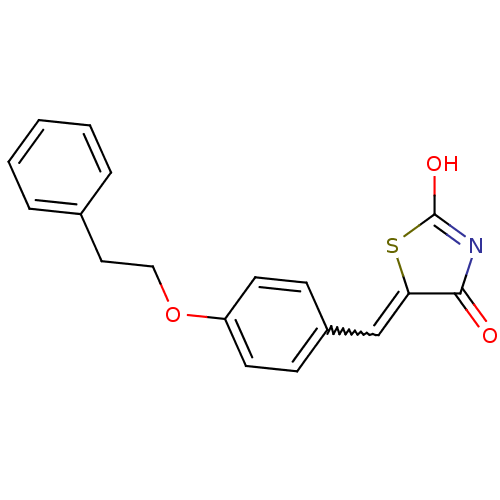 (US8637558, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 117 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118045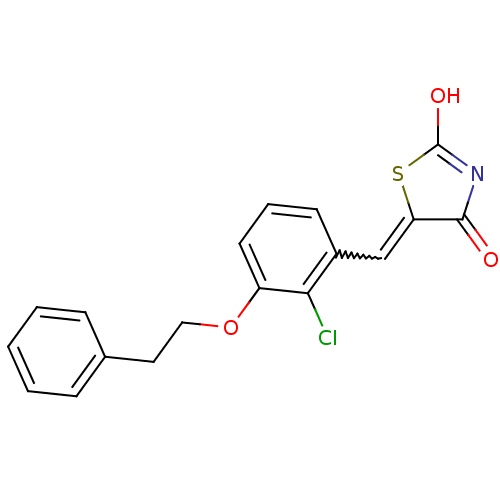 (US8637558, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 122 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378638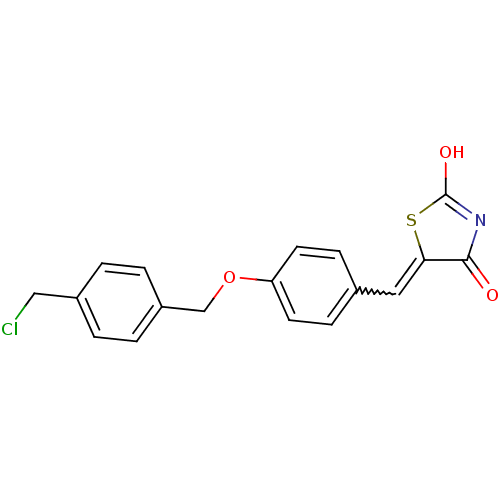 (CHEMBL591466 | US8637558, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118080 (US8637558, 117 | US8637558, 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118059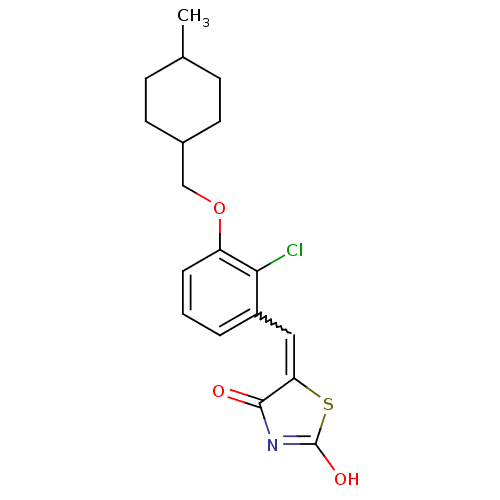 (US8637558, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 135 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118053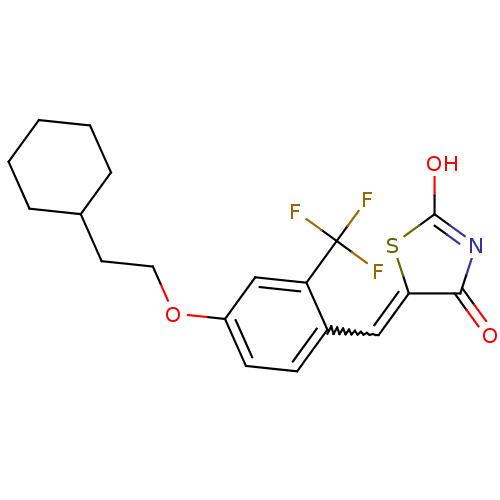 (US8637558, 81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 137 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118085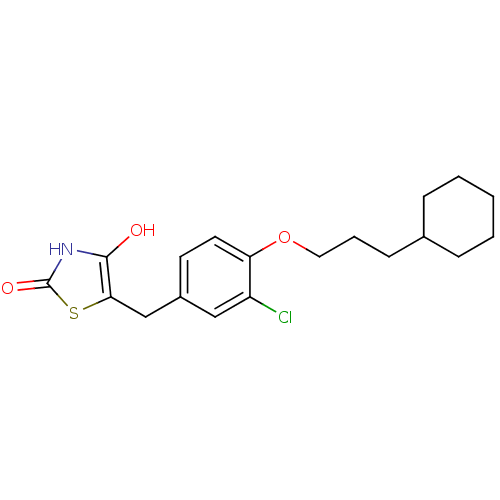 (US8637558, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 143 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM50378621 (CHEMBL610221 | US8637558, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 149 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-hydroxyprostaglandin dehydrogenase [NAD(+)] (Homo sapiens (Human)) | BDBM118072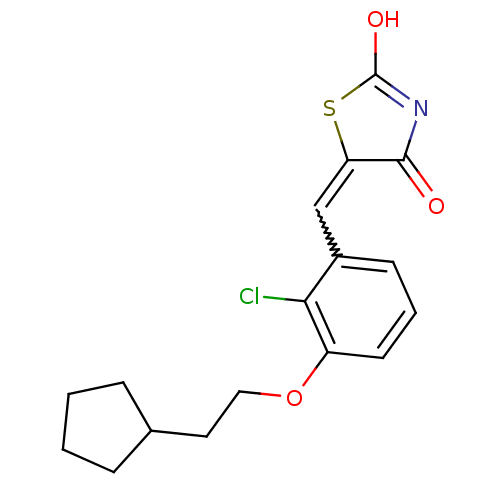 (US8637558, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Industry-Academic Cooperation Foundation, Chosun University US Patent | Assay Description Experimental was performed by measuring the formation of NADH at 340 nm with a fluorescence spectrophotometer. Specifically, 2 ml (in total) of the s... | US Patent US8637558 (2014) BindingDB Entry DOI: 10.7270/Q280519F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 130 total ) | Next | Last >> |