

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM234385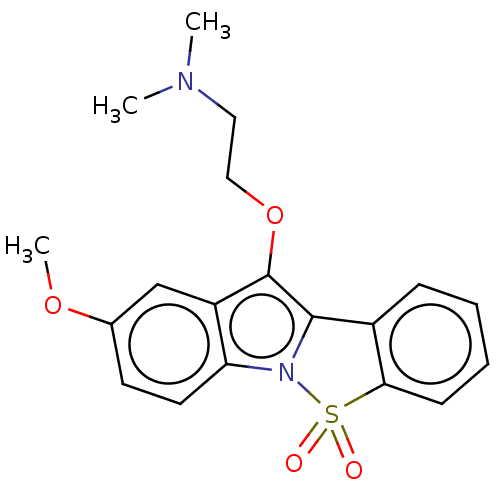 (2-Methoxy-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 127 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Suven Life Sciences Ltd | Assay Description Briefly, receptor source and radioligand used were human recombinant expressed in HEK-293 cells and [3H] LSD (60–80 Ci/mmol), respectively. The ... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM234384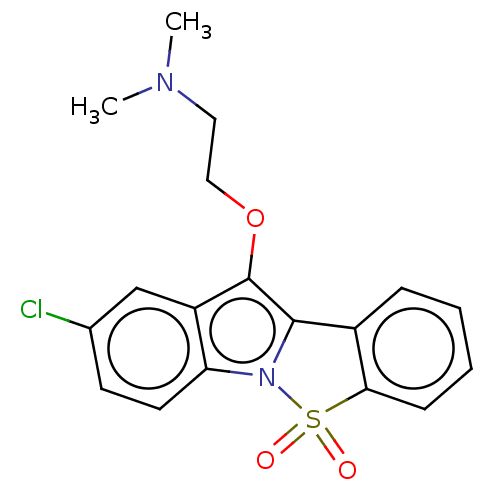 (2-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 464 | -37.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Suven Life Sciences Ltd | Assay Description Briefly, receptor source and radioligand used were human recombinant expressed in HEK-293 cells and [3H] LSD (60–80 Ci/mmol), respectively. The ... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM234386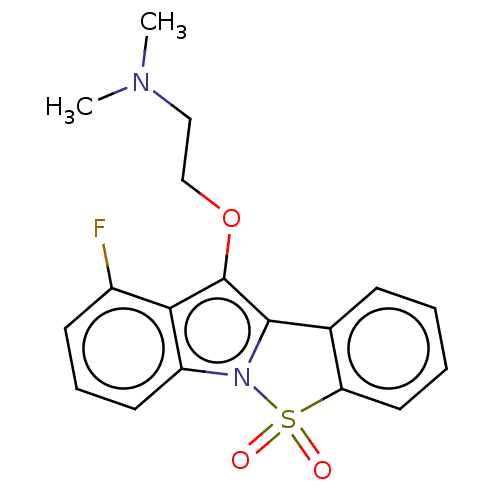 (1-Fluoro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >500 | >-37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Suven Life Sciences Ltd | Assay Description Briefly, receptor source and radioligand used were human recombinant expressed in HEK-293 cells and [3H] LSD (60–80 Ci/mmol), respectively. The ... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM234388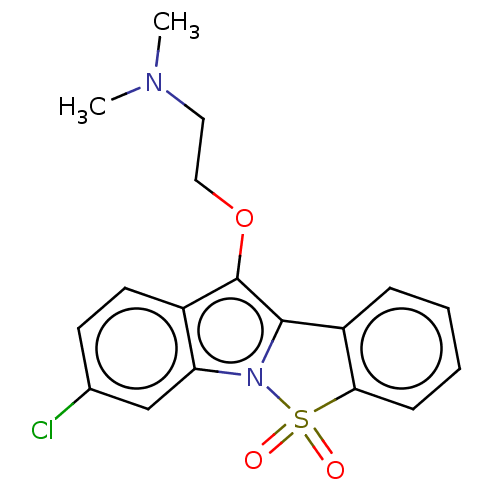 (3-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description The cytochrome P450 inhibitory potential was determined using isoform-selective assays and heterologously expressed human CYP2D6 and CYP3A4. | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM234387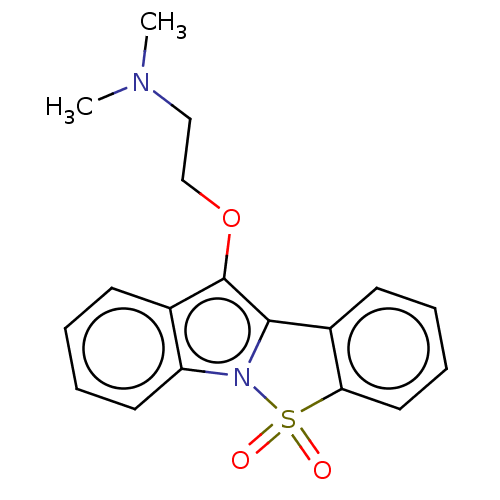 (10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b-azainden...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description The cytochrome P450 inhibitory potential was determined using isoform-selective assays and heterologously expressed human CYP2D6 and CYP3A4. | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM234388 (3-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description Muscarinic acetylcholine receptor M1 was cloned into an expression vector and transfected in CHO cells along with CRE-Luc reporter system. Isolated c... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM234387 (10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b-azainden...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description Muscarinic acetylcholine receptor M1 was cloned into an expression vector and transfected in CHO cells along with CRE-Luc reporter system. Isolated c... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM234387 (10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b-azainden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description Recombinant CHO cells were established by transfecting an expression vector encoding human 5-HT4a gene and CRE-Luc reporter system. Isolated colonies... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM234388 (3-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description CHO cells were transfected with an expression vector encoding rat 5-HT7 gene and CRE-Luc reporter system. Colonies were isolated and screened with 10... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Rattus norvegicus (rat)) | BDBM234387 (10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b-azainden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description CHO cells were transfected with an expression vector encoding rat 5-HT7 gene and CRE-Luc reporter system. Colonies were isolated and screened with 10... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM234388 (3-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description Recombinant CHO cells were established by transfecting an expression vector encoding human 5-HT4a gene and CRE-Luc reporter system. Isolated colonies... | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM234388 (3-Chloro-10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description The cytochrome P450 inhibitory potential was determined using isoform-selective assays and heterologously expressed human CYP2D6 and CYP3A4. | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM234387 (10-(2-N,N-Dimethylaminoethoxy)-5-Thia-4-b-azainden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd | Assay Description The cytochrome P450 inhibitory potential was determined using isoform-selective assays and heterologously expressed human CYP2D6 and CYP3A4. | J Enzyme Inhib Med Chem 26: 341-9 (2011) Article DOI: 10.3109/14756366.2010.510471 BindingDB Entry DOI: 10.7270/Q2WW7GHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||