 Found 15 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50004934
Found 15 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 50004934 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against mutant Y50F scytalone dehydratase |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50507492

(Loxo-195 | Selitrectinib | US10966985, Compound 33...)Show SMILES C[C@@H]1CCc2ncc(F)cc2[C@H]2CCCN2c2ccn3ncc(C(=O)N1)c3n2 Show InChI InChI=1S/C20H21FN6O/c1-12-4-5-16-14(9-13(21)10-22-16)17-3-2-7-26(17)18-6-8-27-19(25-18)15(11-23-27)20(28)24-12/h6,8-12,17H,2-5,7H2,1H3,(H,24,28)/t12-,17-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity against wild type scytalone dehydratase (SD) |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM374727
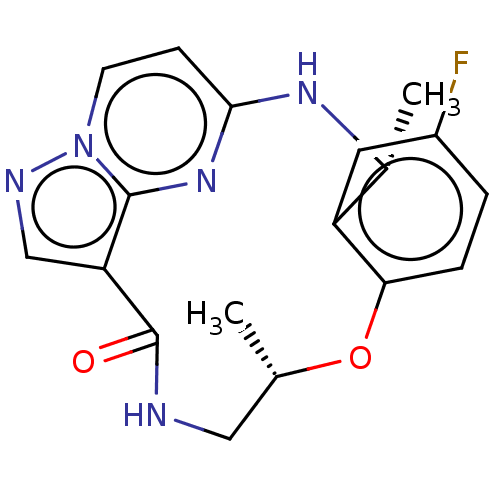
((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...)Show SMILES C[C@H]1CNC(=O)c2cnn3ccc(N[C@H](C)c4cc(F)ccc4O1)nc23 |r| Show InChI InChI=1S/C18H18FN5O2/c1-10-8-20-18(25)14-9-21-24-6-5-16(23-17(14)24)22-11(2)13-7-12(19)3-4-15(13)26-10/h3-7,9-11H,8H2,1-2H3,(H,20,25)(H,22,23)/t10-,11+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against NS3 protease |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224533

(CHEMBL5266269)Show SMILES COc1ccc(OCC(O)CNC(C)COC(C)COC(C)COC(C)COC(C)COC(C)COCC(C)N)cc1 Show InChI InChI=1S/C31H58N2O9/c1-22(32)14-36-16-24(3)38-18-26(5)40-20-28(7)41-19-27(6)39-17-25(4)37-15-23(2)33-13-29(34)21-42-31-11-9-30(35-8)10-12-31/h9-12,22-29,33-34H,13-21,32H2,1-8H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Escherichia coli |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM136597
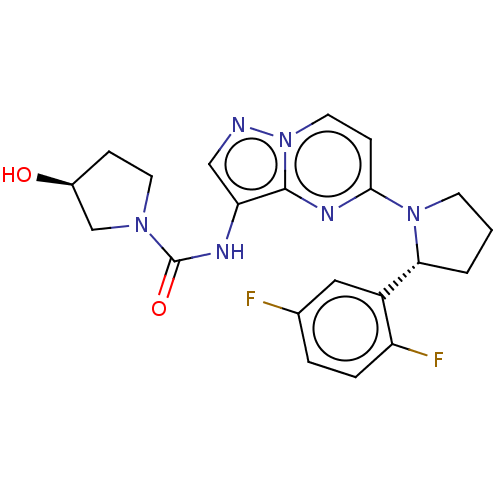
(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Compound was tested for the contractile effects antagonized by mepyramine (1-1000 nM) against Histamine H1 receptor of guinea pig aorta. |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224532
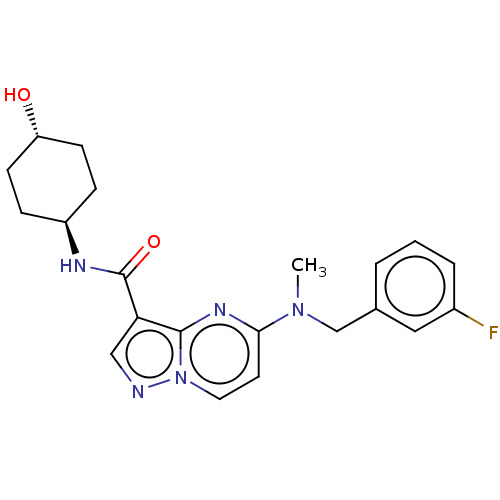
(CHEMBL5282675)Show SMILES CC(N)COCC(C)OCC(C)OCC(C)OCC(C)OCC(C)OCC(C)NCC(O)COc1cccc2ccccc12 Show InChI InChI=1S/C34H58N2O8/c1-24(35)16-38-18-26(3)40-20-28(5)42-22-30(7)43-21-29(6)41-19-27(4)39-17-25(2)36-15-32(37)23-44-34-14-10-12-31-11-8-9-13-33(31)34/h8-14,24-30,32,36-37H,15-23,35H2,1-7H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Escherichia coli |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224528
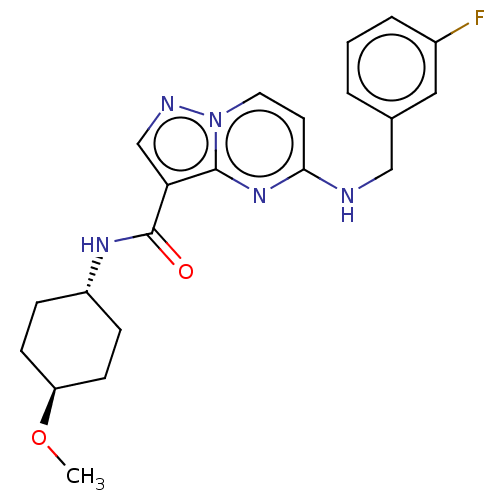
(CHEMBL5275171)Show SMILES COc1ccc(OCC(O)CNC(C)COCC(C)OCC(C)OCC(C)N(CC(O)COc2ccc(OC)cc2)CC(O)COc2ccc(OC)cc2)cc1 Show InChI InChI=1S/C42H64N2O12/c1-30(43-20-34(45)27-54-40-14-8-37(48-5)9-15-40)23-51-25-32(3)53-26-33(4)52-24-31(2)44(21-35(46)28-55-41-16-10-38(49-6)11-17-41)22-36(47)29-56-42-18-12-39(50-7)13-19-42/h8-19,30-36,43,45-47H,20-29H2,1-7H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224527

(CHEMBL5288589)Show SMILES CC(COCC(C)OCC(C)OCC(C)NCC(O)COc1ccccc1CC=C)NCC(O)COc1ccccc1CC=C Show InChI InChI=1S/C36H56N2O7/c1-7-13-31-15-9-11-17-35(31)44-25-33(39)19-37-27(3)21-41-23-29(5)43-24-30(6)42-22-28(4)38-20-34(40)26-45-36-18-12-10-16-32(36)14-8-2/h7-12,15-18,27-30,33-34,37-40H,1-2,13-14,19-26H2,3-6H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224529

(CHEMBL5287060)Show SMILES CC(COCC(C)N(CC(O)COc1cccc2ccccc12)CC(O)COc1cccc2ccccc12)OCC(C)OCC(C)N(CC(O)COc1cccc2ccccc12)CC(O)COc1cccc2ccccc12 Show InChI InChI=1S/C64H76N2O11/c1-45(65(33-53(67)41-74-61-29-13-21-49-17-5-9-25-57(49)61)34-54(68)42-75-62-30-14-22-50-18-6-10-26-58(50)62)37-71-39-47(3)73-40-48(4)72-38-46(2)66(35-55(69)43-76-63-31-15-23-51-19-7-11-27-59(51)63)36-56(70)44-77-64-32-16-24-52-20-8-12-28-60(52)64/h5-32,45-48,53-56,67-70H,33-44H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224553
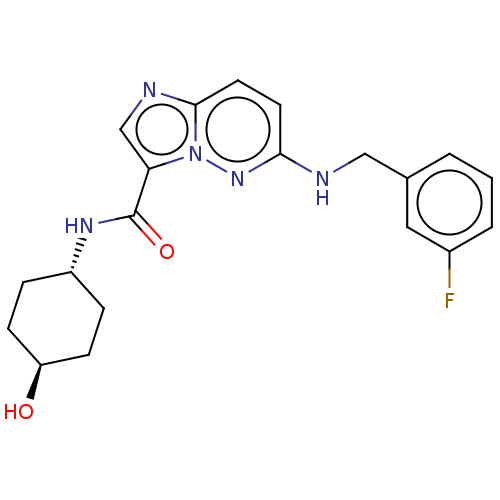
(CHEMBL5287063)Show SMILES CC(COCC(C)OCC(C)OCC(C)N(CC(O)COc1ccccc1CC=C)CC(O)COc1ccccc1CC=C)NCC(O)COc1ccccc1CC=C Show InChI InChI=1S/C48H70N2O9/c1-8-17-40-20-11-14-23-46(40)57-33-43(51)26-49-36(4)29-54-31-38(6)56-32-39(7)55-30-37(5)50(27-44(52)34-58-47-24-15-12-21-41(47)18-9-2)28-45(53)35-59-48-25-16-13-22-42(48)19-10-3/h8-16,20-25,36-39,43-45,49,51-53H,1-3,17-19,26-35H2,4-7H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Escherichia coli |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50225315
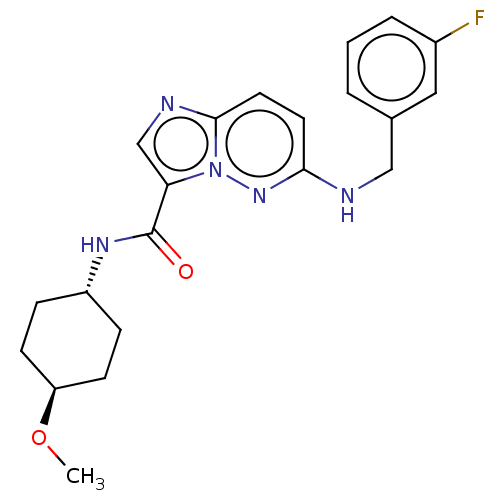
(CHEMBL5287847)Show SMILES Br.[H][C@@]12Cc3ccc(OC)cc3[C@]3(CCCCC13)CCN2C |TLB:10:11:17:18.19.20,16:17:18.19.20:4.3.11,THB:21:20:17:4.3.11| Show InChI InChI=1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15?,17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Escherichia coli |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224530
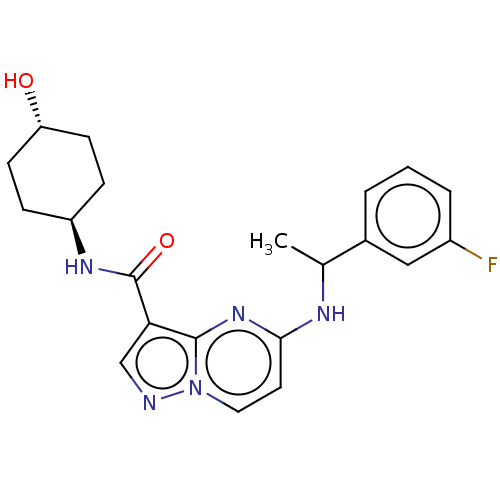
(CHEMBL5291020)Show SMILES CC(COCC(C)OCC(C)OCC(C)N(CC(O)COc1cccc2ccccc12)CC(O)COc1cccc2ccccc12)NCC(O)COc1cccc2ccccc12 Show InChI InChI=1S/C51H64N2O9/c1-36(52-26-43(54)33-60-49-23-11-17-40-14-5-8-20-46(40)49)29-57-31-38(3)59-32-39(4)58-30-37(2)53(27-44(55)34-61-50-24-12-18-41-15-6-9-21-47(41)50)28-45(56)35-62-51-25-13-19-42-16-7-10-22-48(42)51/h5-25,36-39,43-45,52,54-56H,26-35H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224531

(CHEMBL5275881)Show SMILES COc1ccc(OCC(O)CN(CC(O)COc2ccc(OC)cc2)C(C)COCC(C)OCC(C)OCC(C)N(CC(O)COc2ccc(OC)cc2)CC(O)COc2ccc(OC)cc2)cc1 Show InChI InChI=1S/C52H76N2O15/c1-37(53(25-41(55)33-66-49-17-9-45(59-5)10-18-49)26-42(56)34-67-50-19-11-46(60-6)12-20-50)29-63-31-39(3)65-32-40(4)64-30-38(2)54(27-43(57)35-68-51-21-13-47(61-7)14-22-51)28-44(58)36-69-52-23-15-48(62-8)16-24-52/h9-24,37-44,55-58H,25-36H2,1-8H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224526

(CHEMBL5271403)Show SMILES COCCc1ccc(OCC(O)CNC(C)COC(C)COC(C)COC(C)COC(C)COC(C)COCC(C)N)cc1 Show InChI InChI=1S/C33H62N2O9/c1-24(34)16-38-18-26(3)40-20-28(5)42-22-30(7)43-21-29(6)41-19-27(4)39-17-25(2)35-15-32(36)23-44-33-11-9-31(10-12-33)13-14-37-8/h9-12,24-30,32,35-36H,13-23,34H2,1-8H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Vasopressin V2 receptor antagonistic activity in vivo in anesthetized rats |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50224552

(CHEMBL5291305)Show SMILES CC(COCC(C)N(CC(O)COc1ccccc1CC=C)CC(O)COc1ccccc1CC=C)OCC(C)OCC(C)N(CC(O)COc1ccccc1CC=C)CC(O)COc1ccccc1CC=C Show InChI InChI=1S/C60H84N2O11/c1-9-21-49-25-13-17-29-57(49)70-41-53(63)33-61(34-54(64)42-71-58-30-18-14-26-50(58)22-10-2)45(5)37-67-39-47(7)69-40-48(8)68-38-46(6)62(35-55(65)43-72-59-31-19-15-27-51(59)23-11-3)36-56(66)44-73-60-32-20-16-28-52(60)24-12-4/h9-20,25-32,45-48,53-56,63-66H,1-4,21-24,33-44H2,5-8H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kinki University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dihydrofolate reductase enzyme purified from Escherichia coli |
J Agric Food Chem 53: 5549-53 (2005)
Article DOI: 10.1021/jf050110v |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data