 Found 60 hits of ec50 for UniProtKB: P03372
Found 60 hits of ec50 for UniProtKB: P03372 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471081
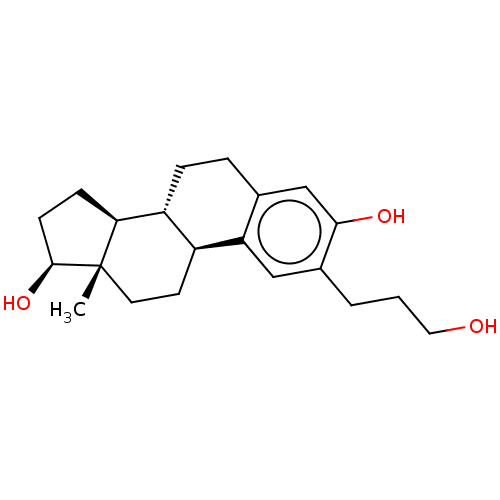
(CHEMBL1627420)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CCCO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C21H30O3/c1-21-9-8-15-16(18(21)6-7-20(21)24)5-4-13-12-19(23)14(3-2-10-22)11-17(13)15/h11-12,15-16,18,20,22-24H,2-10H2,1H3/t15-,16+,18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by ChEMBL
| Assay Description
Percent agonistic activity for estrogen-induced pS2 expression in MCF-7 cells |
Bioorg Med Chem Lett 13: 1919-22 (2003)
BindingDB Entry DOI: 10.7270/Q29K49MK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0301 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 Gene expression in human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.0996 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.292 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474892

(CHEMBL184958)Show SMILES OC(CCN1CCCCC1)c1ccc(cc1)C1c2c(Cc3cc(O)ccc13)sc1cc(O)ccc21 Show InChI InChI=1S/C30H31NO3S/c32-22-8-10-24-21(16-22)17-28-30(25-11-9-23(33)18-27(25)35-28)29(24)20-6-4-19(5-7-20)26(34)12-15-31-13-2-1-3-14-31/h4-11,16,18,26,29,32-34H,1-3,12-15,17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474885
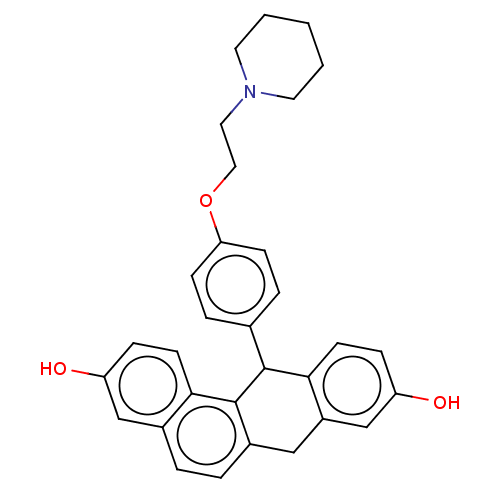
(CHEMBL360872)Show SMILES Oc1ccc2C(c3ccc(OCCN4CCCCC4)cc3)c3c(Cc2c1)ccc1cc(O)ccc31 Show InChI InChI=1S/C31H31NO3/c33-25-8-12-28-22(19-25)4-5-23-18-24-20-26(34)9-13-29(24)30(31(23)28)21-6-10-27(11-7-21)35-17-16-32-14-2-1-3-15-32/h4-13,19-20,30,33-34H,1-3,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474893
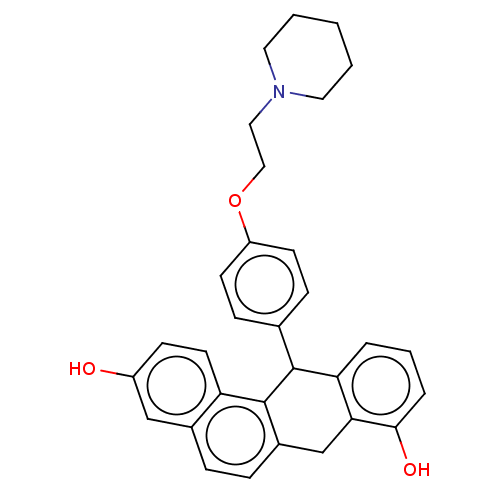
(CHEMBL184976)Show SMILES Oc1ccc2c3C(c4ccc(OCCN5CCCCC5)cc4)c4cccc(O)c4Cc3ccc2c1 Show InChI InChI=1S/C31H31NO3/c33-24-11-14-26-22(19-24)7-8-23-20-28-27(5-4-6-29(28)34)30(31(23)26)21-9-12-25(13-10-21)35-18-17-32-15-2-1-3-16-32/h4-14,19,30,33-34H,1-3,15-18,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50276802
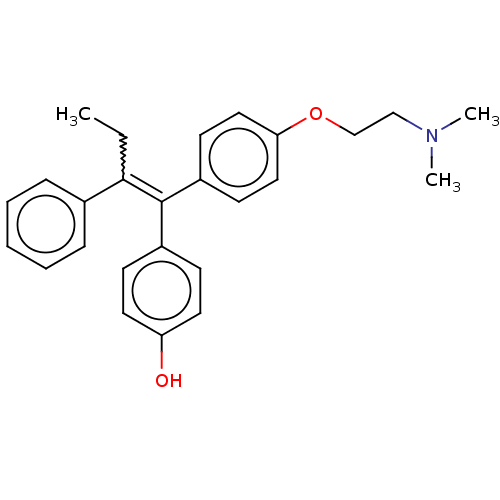
(4-OHT | Afimoxifene | TamoGel)Show SMILES CCC(=C(c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474894

(CHEMBL185412)Show SMILES OC(CCN1CCCCC1)c1ccc(cc1)C1c2c(Cc3ccc(O)cc13)sc1cc(O)ccc21 Show InChI InChI=1S/C30H31NO3S/c32-22-9-8-21-16-28-30(24-11-10-23(33)18-27(24)35-28)29(25(21)17-22)20-6-4-19(5-7-20)26(34)12-15-31-13-2-1-3-14-31/h4-11,17-18,26,29,32-34H,1-3,12-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.996 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Boston University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor-ligand binding domain |
Bioorg Med Chem Lett 9: 2379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2CC12V7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474886
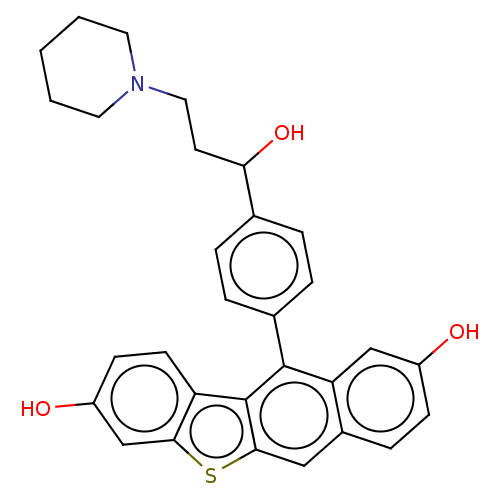
(CHEMBL182342)Show SMILES OC(CCN1CCCCC1)c1ccc(cc1)-c1c2c(cc3ccc(O)cc13)sc1cc(O)ccc21 Show InChI InChI=1S/C30H29NO3S/c32-22-9-8-21-16-28-30(24-11-10-23(33)18-27(24)35-28)29(25(21)17-22)20-6-4-19(5-7-20)26(34)12-15-31-13-2-1-3-14-31/h4-11,16-18,26,32-34H,1-3,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474891
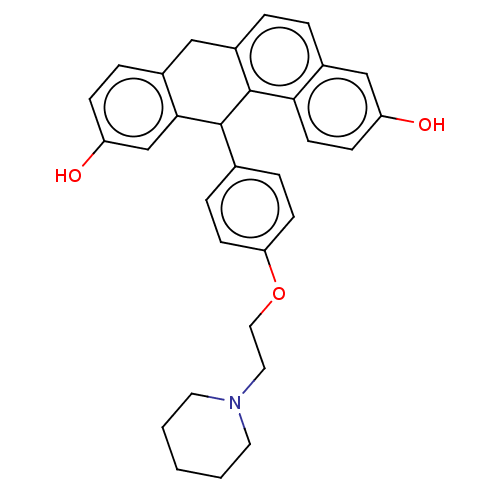
(CHEMBL184423)Show SMILES Oc1ccc2Cc3ccc4cc(O)ccc4c3C(c3ccc(OCCN4CCCCC4)cc3)c2c1 Show InChI InChI=1S/C31H31NO3/c33-25-10-13-28-23(19-25)4-5-24-18-22-6-9-26(34)20-29(22)30(31(24)28)21-7-11-27(12-8-21)35-17-16-32-14-2-1-3-15-32/h4-13,19-20,30,33-34H,1-3,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471080
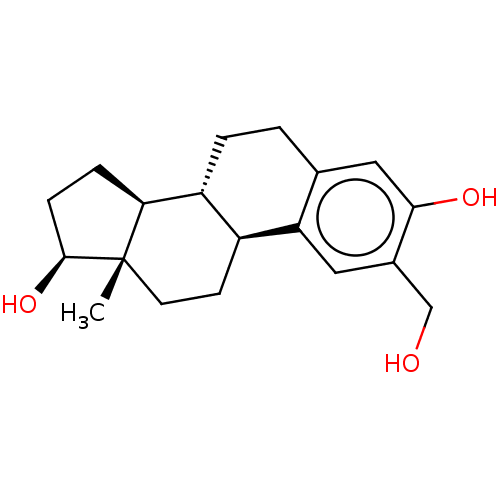
(CHEMBL1627431)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-9,13-14,16,18,20-22H,2-7,10H2,1H3/t13-,14+,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474887
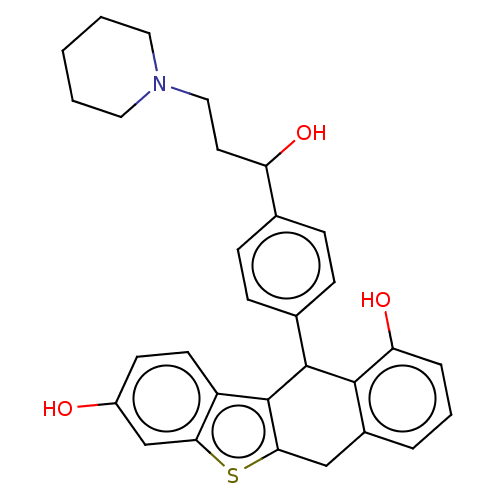
(CHEMBL185284)Show SMILES OC(CCN1CCCCC1)c1ccc(cc1)C1c2c(Cc3cccc(O)c13)sc1cc(O)ccc21 Show InChI InChI=1S/C30H31NO3S/c32-22-11-12-23-26(18-22)35-27-17-21-5-4-6-25(34)28(21)29(30(23)27)20-9-7-19(8-10-20)24(33)13-16-31-14-2-1-3-15-31/h4-12,18,24,29,32-34H,1-3,13-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474885

(CHEMBL360872)Show SMILES Oc1ccc2C(c3ccc(OCCN4CCCCC4)cc3)c3c(Cc2c1)ccc1cc(O)ccc31 Show InChI InChI=1S/C31H31NO3/c33-25-8-12-28-22(19-25)4-5-23-18-24-20-26(34)9-13-29(24)30(31(23)28)21-6-10-27(11-7-21)35-17-16-32-14-2-1-3-15-32/h4-13,19-20,30,33-34H,1-3,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474885

(CHEMBL360872)Show SMILES Oc1ccc2C(c3ccc(OCCN4CCCCC4)cc3)c3c(Cc2c1)ccc1cc(O)ccc31 Show InChI InChI=1S/C31H31NO3/c33-25-8-12-28-22(19-25)4-5-23-18-24-20-26(34)9-13-29(24)30(31(23)28)21-6-10-27(11-7-21)35-17-16-32-14-2-1-3-15-32/h4-13,19-20,30,33-34H,1-3,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474889
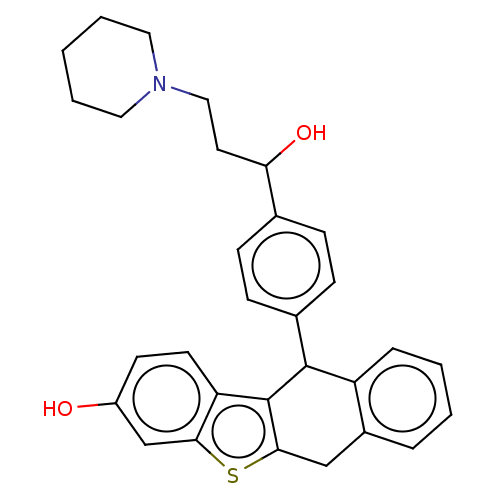
(CHEMBL185064)Show SMILES OC(CCN1CCCCC1)c1ccc(cc1)C1c2c(Cc3ccccc13)sc1cc(O)ccc21 Show InChI InChI=1S/C30H31NO2S/c32-23-12-13-25-27(19-23)34-28-18-22-6-2-3-7-24(22)29(30(25)28)21-10-8-20(9-11-21)26(33)14-17-31-15-4-1-5-16-31/h2-3,6-13,19,26,29,32-33H,1,4-5,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity estrogen receptor of MCF-7 human mammary cancer cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471263
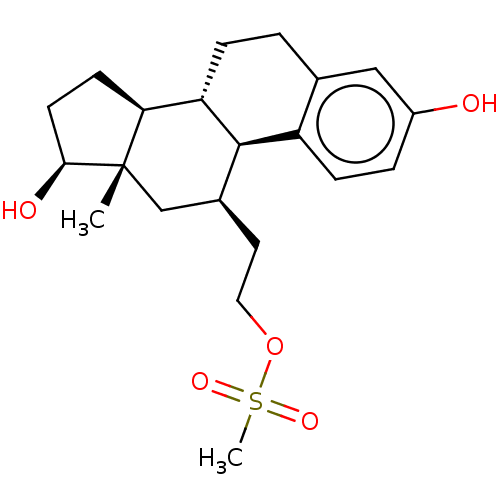
(CHEMBL1627873)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)C[C@H](CCOS(C)(=O)=O)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C21H30O5S/c1-21-12-14(9-10-26-27(2,24)25)20-16-6-4-15(22)11-13(16)3-5-17(20)18(21)7-8-19(21)23/h4,6,11,14,17-20,22-23H,3,5,7-10,12H2,1-2H3/t14-,17-,18-,19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
INSERM Unit� 439
Curated by ChEMBL
| Assay Description
Compound concentration required to induce transcriptional activation in MVLN cells comparable to 50% of 0.1 nM estradiol response |
J Med Chem 40: 2217-27 (1997)
Article DOI: 10.1021/jm970019l
BindingDB Entry DOI: 10.7270/Q20G3NVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471080

(CHEMBL1627431)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-9,13-14,16,18,20-22H,2-7,10H2,1H3/t13-,14+,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471261
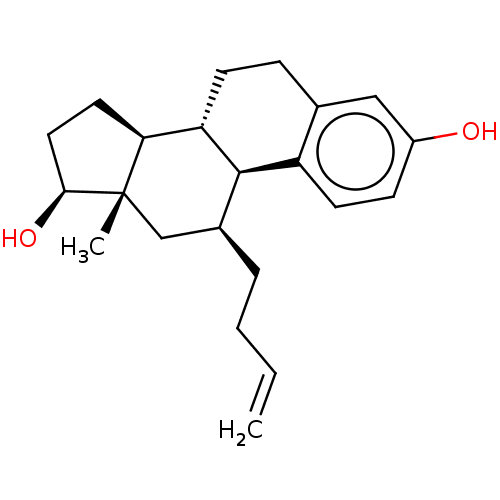
(CHEMBL1627868)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)C[C@H](CCC=C)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C22H30O2/c1-3-4-5-15-13-22(2)19(10-11-20(22)24)18-8-6-14-12-16(23)7-9-17(14)21(15)18/h3,7,9,12,15,18-21,23-24H,1,4-6,8,10-11,13H2,2H3/t15-,18-,19-,20-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a |
INSERM Unit� 439
Curated by ChEMBL
| Assay Description
Compound concentration required to induce transcriptional activation in MVLN cells comparable to 50% of 0.1 nM estradiol response |
J Med Chem 40: 2217-27 (1997)
Article DOI: 10.1021/jm970019l
BindingDB Entry DOI: 10.7270/Q20G3NVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474888
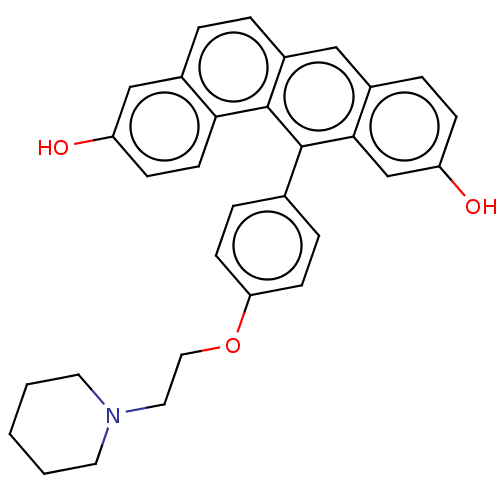
(CHEMBL183685)Show SMILES Oc1ccc2c(ccc3cc4ccc(O)cc4c(-c4ccc(OCCN5CCCCC5)cc4)c23)c1 Show InChI InChI=1S/C31H29NO3/c33-25-10-13-28-23(19-25)4-5-24-18-22-6-9-26(34)20-29(22)30(31(24)28)21-7-11-27(12-8-21)35-17-16-32-14-2-1-3-15-32/h4-13,18-20,33-34H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50128415

(4-((1R,2R,5S)-5-Hydroxymethyl-bicyclo[3.3.1]non-7-...)Show SMILES OC[C@]12CC[C@H]([C@H](C1)C=CC2)c1ccc(O)cc1 |c:9| Show InChI InChI=1S/C16H20O2/c17-11-16-8-1-2-13(10-16)15(7-9-16)12-3-5-14(18)6-4-12/h1-6,13,15,17-18H,7-11H2/t13?,15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | <10 | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by ChEMBL
| Assay Description
Percent agonistic activity for estrogen-induced pS2 expression in MCF-7 cells |
Bioorg Med Chem Lett 13: 1919-22 (2003)
BindingDB Entry DOI: 10.7270/Q29K49MK |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471440
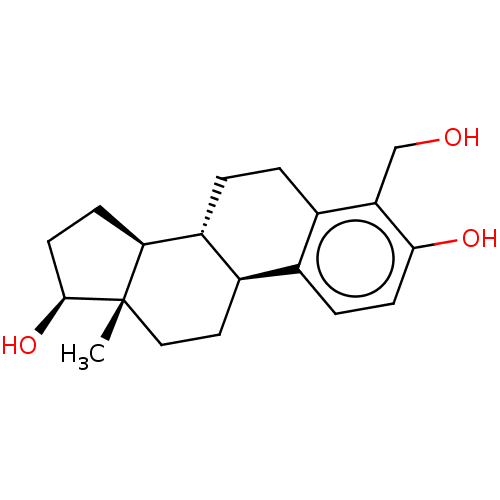
(CHEMBL1627574)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CO)c3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-9-8-13-11-4-6-17(21)15(10-20)12(11)2-3-14(13)16(19)5-7-18(19)22/h4,6,13-14,16,18,20-22H,2-3,5,7-10H2,1H3/t13-,14-,16+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 Gene expression in human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50128414

(4-((1R,5R)-5-Hydroxymethyl-8-methyl-3-oxa-bicyclo[...)Show SMILES CC1=CC[C@@]2(CO)COC([C@@H]1C2)c1ccc(O)cc1 |t:1| Show InChI InChI=1S/C16H20O3/c1-11-6-7-16(9-17)8-14(11)15(19-10-16)12-2-4-13(18)5-3-12/h2-6,14-15,17-18H,7-10H2,1H3/t14?,15?,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bayer Corporation
Curated by ChEMBL
| Assay Description
Percent agonistic activity for estrogen-induced pS2 expression in MCF-7 cells |
Bioorg Med Chem Lett 13: 1919-22 (2003)
BindingDB Entry DOI: 10.7270/Q29K49MK |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50474890
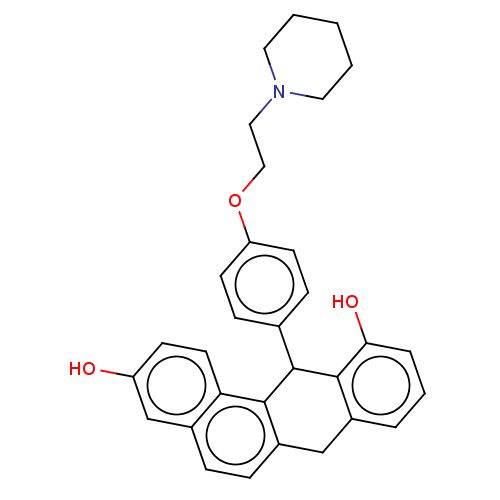
(CHEMBL360005)Show SMILES Oc1ccc2c3C(c4ccc(OCCN5CCCCC5)cc4)c4c(O)cccc4Cc3ccc2c1 Show InChI InChI=1S/C31H31NO3/c33-25-11-14-27-22(20-25)7-8-24-19-23-5-4-6-28(34)30(23)31(29(24)27)21-9-12-26(13-10-21)35-18-17-32-15-2-1-3-16-32/h4-14,20,31,33-34H,1-3,15-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Agonist activity as alkaline phosphatase induction in Ishikawa endometrial cells compared to E2 |
Bioorg Med Chem Lett 14: 5103-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.072
BindingDB Entry DOI: 10.7270/Q25X2CQ6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM34649

((8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c(O)c(O)ccc34)[C@@H]1CC[C@@H]2O Show InChI InChI=1S/C18H24O3/c1-18-9-8-11-10-4-6-15(19)17(21)13(10)3-2-12(11)14(18)5-7-16(18)20/h4,6,11-12,14,16,19-21H,2-3,5,7-9H2,1H3/t11-,12-,14+,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 Gene expression in human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471265
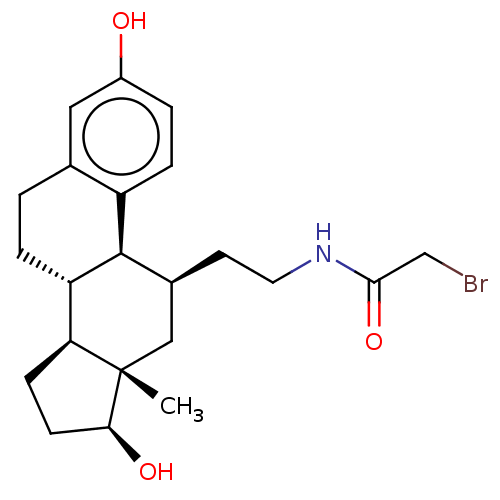
(CHEMBL1627460)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)C[C@H](CCNC(=O)CBr)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C22H30BrNO3/c1-22-11-14(8-9-24-20(27)12-23)21-16-5-3-15(25)10-13(16)2-4-17(21)18(22)6-7-19(22)26/h3,5,10,14,17-19,21,25-26H,2,4,6-9,11-12H2,1H3,(H,24,27)/t14-,17-,18-,19-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
INSERM Unit� 439
Curated by ChEMBL
| Assay Description
Compound concentration required to induce transcriptional activation in MVLN cells comparable to 50% of 0.1 nM estradiol response |
J Med Chem 40: 2217-27 (1997)
Article DOI: 10.1021/jm970019l
BindingDB Entry DOI: 10.7270/Q20G3NVN |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471080

(CHEMBL1627431)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-9,13-14,16,18,20-22H,2-7,10H2,1H3/t13-,14+,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 146 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471439
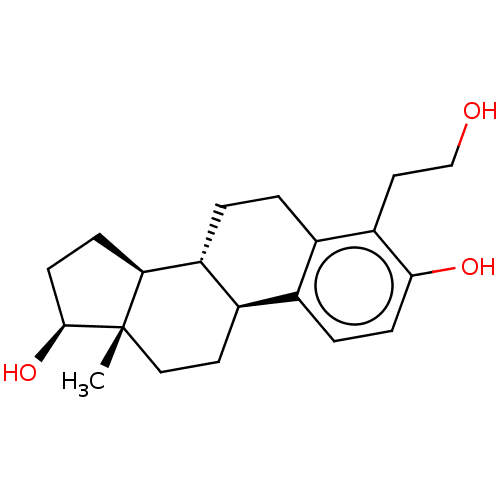
(CHEMBL1627561)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CCO)c3CC[C@@]21[H] Show InChI InChI=1S/C20H28O3/c1-20-10-8-14-12-4-6-18(22)16(9-11-21)13(12)2-3-15(14)17(20)5-7-19(20)23/h4,6,14-15,17,19,21-23H,2-3,5,7-11H2,1H3/t14-,15-,17+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 148 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 Gene expression in human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471079
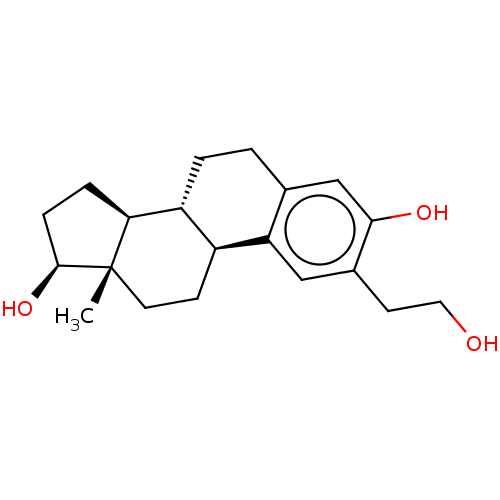
(CHEMBL1628150)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CCO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C20H28O3/c1-20-8-6-14-15(17(20)4-5-19(20)23)3-2-12-11-18(22)13(7-9-21)10-16(12)14/h10-11,14-15,17,19,21-23H,2-9H2,1H3/t14-,15+,17-,19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 272 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50217045
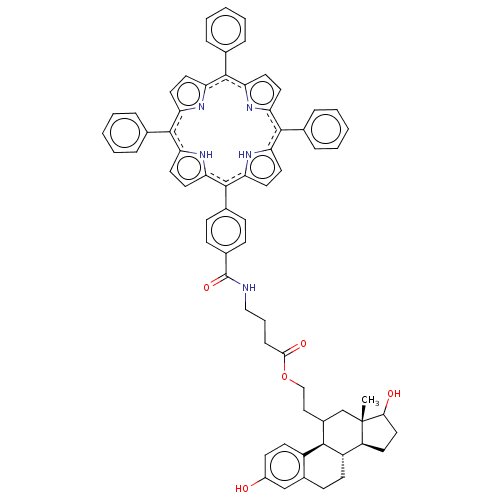
(CHEMBL2068062)Show SMILES [H][C@@]12CCC(O)[C@@]1(C)CC(CCOC(=O)CCCNC(=O)c1ccc(cc1)-c1c3ccc([nH]3)c(-c3ccccc3)c3ccc(n3)c(-c3ccccc3)c3ccc(n3)c(-c3ccccc3)c3ccc1[nH]3)[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C69H63N5O5/c1-69-41-48(63-50-26-24-49(75)40-47(50)23-25-51(63)52(69)27-36-61(69)76)37-39-79-62(77)18-11-38-70-68(78)46-21-19-45(20-22-46)67-59-34-32-57(73-59)65(43-14-7-3-8-15-43)55-30-28-53(71-55)64(42-12-5-2-6-13-42)54-29-31-56(72-54)66(44-16-9-4-10-17-44)58-33-35-60(67)74-58/h2-10,12-17,19-22,24,26,28-35,40,48,51-52,61,63,73-76H,11,18,23,25,27,36-39,41H2,1H3,(H,70,78)/b64-53-,64-54-,65-55-,65-57-,66-56-,66-58-,67-59-,67-60-/t48?,51-,52-,61?,63+,69-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 274 | n/a | n/a | n/a | n/a |
Boston University School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor-ligand binding domain |
Bioorg Med Chem Lett 9: 2379-84 (1999)
BindingDB Entry DOI: 10.7270/Q2CC12V7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471080

(CHEMBL1627431)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-9,13-14,16,18,20-22H,2-7,10H2,1H3/t13-,14+,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 342 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity estrogen receptor of MCF-7 human mammary cancer cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471440

(CHEMBL1627574)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CO)c3CC[C@@]21[H] Show InChI InChI=1S/C19H26O3/c1-19-9-8-13-11-4-6-17(21)15(10-20)12(11)2-3-14(13)16(19)5-7-18(19)22/h4,6,13-14,16,18,20-22H,2-3,5,7-10H2,1H3/t13-,14-,16+,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 364 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471081

(CHEMBL1627420)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(CCCO)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C21H30O3/c1-21-9-8-15-16(18(21)6-7-20(21)24)5-4-13-12-19(23)14(3-2-10-22)11-17(13)15/h11-12,15-16,18,20,22-24H,2-10H2,1H3/t15-,16+,18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 495 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50262140
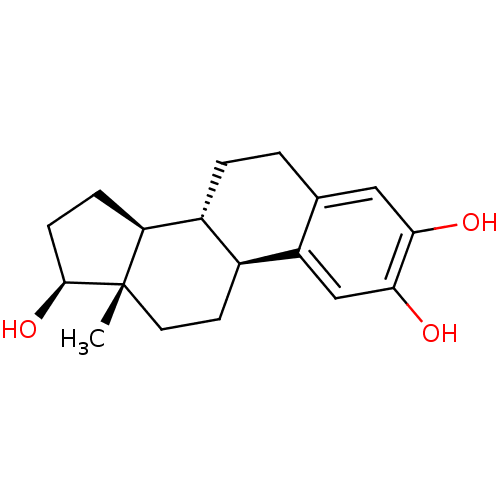
((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(O)cc34)[C@@H]1CC[C@@H]2O |r| Show InChI InChI=1S/C18H24O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,17,19-21H,2-7H2,1H3/t11-,12+,14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 504 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 mRNA expression in human MCF-7 mammary carcinoma cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM34649

((8R,9S,13S,14S,17S)-13-methyl-6,7,8,9,11,12,14,15,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c(O)c(O)ccc34)[C@@H]1CC[C@@H]2O Show InChI InChI=1S/C18H24O3/c1-18-9-8-11-10-4-6-15(19)17(21)13(10)3-2-12(11)14(18)5-7-16(18)20/h4,6,11-12,14,16,19-21H,2-3,5,7-9H2,1H3/t11-,12-,14+,16+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 506 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471439

(CHEMBL1627561)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CCO)c3CC[C@@]21[H] Show InChI InChI=1S/C20H28O3/c1-20-10-8-14-12-4-6-18(22)16(9-11-21)13(12)2-3-15(14)17(20)5-7-19(20)23/h4,6,14-15,17,19,21-23H,2-3,5,7-11H2,1H3/t14-,15-,17+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471082
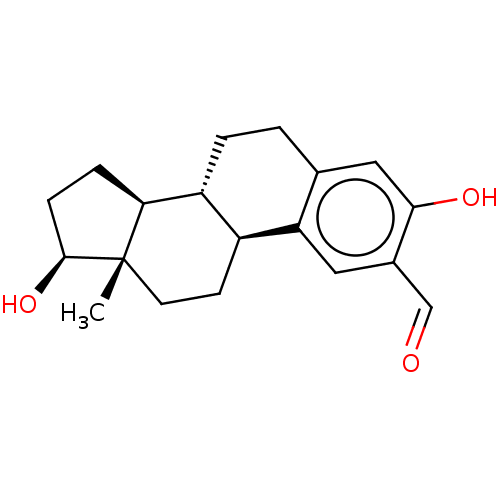
(CHEMBL1627841)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3cc(C=O)c(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C19H24O3/c1-19-7-6-13-14(16(19)4-5-18(19)22)3-2-11-9-17(21)12(10-20)8-15(11)13/h8-10,13-14,16,18,21-22H,2-7H2,1H3/t13-,14+,16-,18-,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 782 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity estrogen receptor of MCF-7 human mammary cancer cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50262140

((17beta)-estra-1,3,5(10)-triene-2,3,17-triol | 2-O...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)c(O)cc34)[C@@H]1CC[C@@H]2O |r| Show InChI InChI=1S/C18H24O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,17,19-21H,2-7H2,1H3/t11-,12+,14-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity estrogen receptor of MCF-7 human mammary cancer cells |
J Med Chem 39: 1917-23 (1996)
Article DOI: 10.1021/jm9508245
BindingDB Entry DOI: 10.7270/Q21G0Q0F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in immature rabbit |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471441
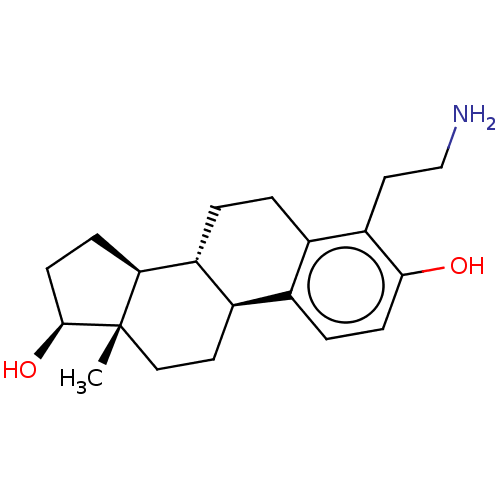
(CHEMBL1627560)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CCN)c3CC[C@@]21[H] Show InChI InChI=1S/C20H29NO2/c1-20-10-8-14-12-4-6-18(22)16(9-11-21)13(12)2-3-15(14)17(20)5-7-19(20)23/h4,6,14-15,17,19,22-23H,2-3,5,7-11,21H2,1H3/t14-,15-,17+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells at 10e-5 M |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471442
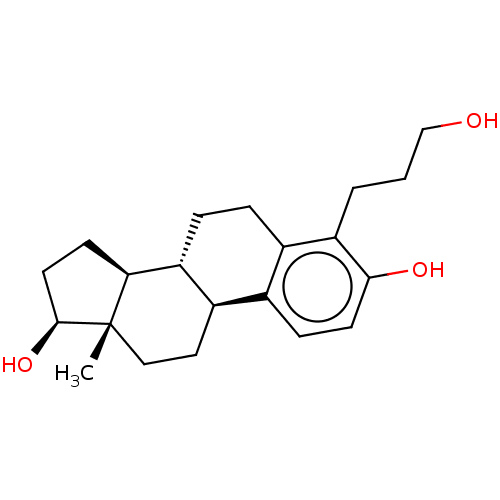
(CHEMBL1627932)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CCCO)c3CC[C@@]21[H] Show InChI InChI=1S/C21H30O3/c1-21-11-10-15-13-6-8-19(23)17(3-2-12-22)14(13)4-5-16(15)18(21)7-9-20(21)24/h6,8,15-16,18,20,22-24H,2-5,7,9-12H2,1H3/t15-,16-,18+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Induction of pS2 Gene expression in human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50471442

(CHEMBL1627932)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(CCCO)c3CC[C@@]21[H] Show InChI InChI=1S/C21H30O3/c1-21-11-10-15-13-6-8-19(23)17(3-2-12-22)14(13)4-5-16(15)18(21)7-9-20(21)24/h6,8,15-16,18,20,22-24H,2-5,7,9-12H2,1H3/t15-,16-,18+,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.32E+3 | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Affinity for estrogen receptor of human MCF-7 cells |
J Med Chem 40: 3756-64 (1997)
Article DOI: 10.1021/jm9701684
BindingDB Entry DOI: 10.7270/Q2280B95 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from estrogen receptor in squirrel monkey |
J Med Chem 32: 1642-52 (1989)
BindingDB Entry DOI: 10.7270/Q25141F8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data