

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM275908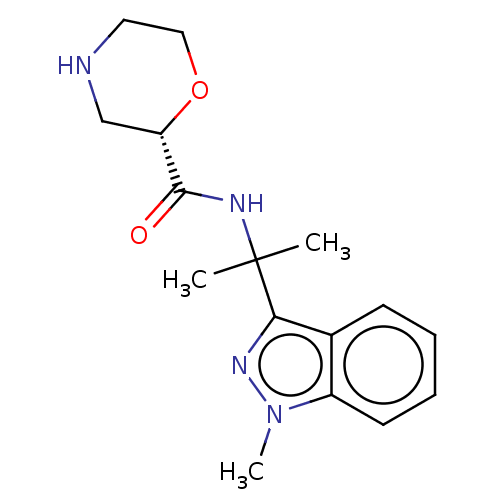 (US10071974, Example 19 | US10577336, Example 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 194 | -38.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10071974 (2018) BindingDB Entry DOI: 10.7270/Q28G8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 194 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10577336 (2020) BindingDB Entry DOI: 10.7270/Q24F1T4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10577336 (2020) BindingDB Entry DOI: 10.7270/Q24F1T4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.71E+3 | -28.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10071974 (2018) BindingDB Entry DOI: 10.7270/Q28G8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.01E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10071974 (2018) BindingDB Entry DOI: 10.7270/Q28G8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 1 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10577336 (2020) BindingDB Entry DOI: 10.7270/Q24F1T4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.63E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10071974 (2018) BindingDB Entry DOI: 10.7270/Q28G8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10577336 (2020) BindingDB Entry DOI: 10.7270/Q24F1T4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Receptor binding assays refer to a technique in which labeled receptor ligands are used to detect binding to a receptor. In competition experiments t... | US Patent US10577336 (2020) BindingDB Entry DOI: 10.7270/Q24F1T4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM275908 (US10071974, Example 19 | US10577336, Example 19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.86E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Boehringer Ingelheim International GmbH US Patent | Assay Description For the binding experiments 200 μL of membrane homogenate from one of the following protein amounts is used: hSSTR1 (40 μg/well); hSSTR2 (2... | US Patent US10071974 (2018) BindingDB Entry DOI: 10.7270/Q28G8NQ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||