 Found 8 hits of ki for monomerid = 50142887
Found 8 hits of ki for monomerid = 50142887 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50142887
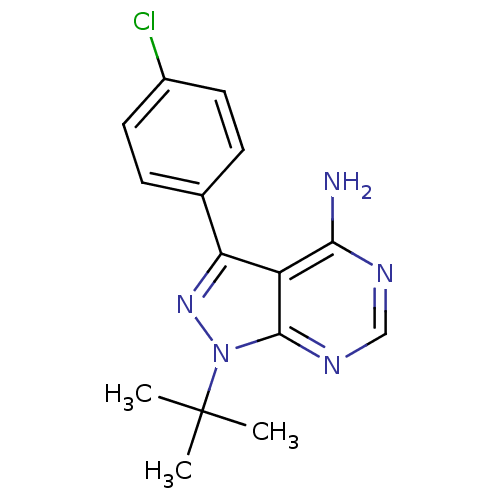
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by PDSP Ki Database
| |
Biochem J 371: 199-204 (2003)
Article DOI: 10.1042/BJ20021535
BindingDB Entry DOI: 10.7270/Q2KH0KV9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibitory activity against Src in cell free assay |
J Med Chem 49: 1549-61 (2006)
Article DOI: 10.1021/jm050603r
BindingDB Entry DOI: 10.7270/Q27H1J6N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant Abl by cell free assay |
J Med Chem 51: 1252-9 (2008)
Article DOI: 10.1021/jm701240c
BindingDB Entry DOI: 10.7270/Q2MW2J10 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Src |
J Med Chem 50: 5579-88 (2007)
Article DOI: 10.1021/jm061449r
BindingDB Entry DOI: 10.7270/Q2HX1CDW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by PDSP Ki Database
| |
Biochem J 371: 199-204 (2003)
Article DOI: 10.1042/BJ20021535
BindingDB Entry DOI: 10.7270/Q2KH0KV9 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Rattus norvegicus (rat)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by PDSP Ki Database
| |
Biochem J 371: 199-204 (2003)
Article DOI: 10.1042/BJ20021535
BindingDB Entry DOI: 10.7270/Q2KH0KV9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50142887

(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
ACS Med Chem Lett 3: 383-386 (2012)
Article DOI: 10.1021/ml300012r
BindingDB Entry DOI: 10.7270/Q2B56KTH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data