 Found 7 hits of ki for monomerid = 50296782
Found 7 hits of ki for monomerid = 50296782 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50296782
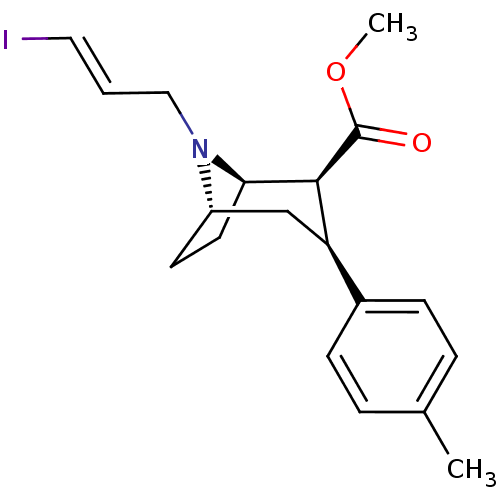
((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Displacement of [125I]PE2I from rat DAT |
Bioorg Med Chem Lett 19: 4843-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.032
BindingDB Entry DOI: 10.7270/Q2VH5NWZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN35428 from human cloned DAT receptor expressed in human HEK293 cells |
Bioorg Med Chem 20: 1388-95 (2012)
Article DOI: 10.1016/j.bmc.2012.01.014
BindingDB Entry DOI: 10.7270/Q26T0N3Z |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna
Curated by ChEMBL
| Assay Description
Binding affinity to DAT (unknown origin) |
Bioorg Med Chem 21: 7562-9 (2013)
Article DOI: 10.1016/j.bmc.2013.10.046
BindingDB Entry DOI: 10.7270/Q27P92BD |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Tours
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 317: 147-52 (2006)
Article DOI: 10.1124/jpet.105.096792
BindingDB Entry DOI: 10.7270/Q2JW8CFZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem 21: 7562-9 (2013)
Article DOI: 10.1016/j.bmc.2013.10.046
BindingDB Entry DOI: 10.7270/Q27P92BD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human cloned NET receptor expressed in HEK293 cells |
Bioorg Med Chem 20: 1388-95 (2012)
Article DOI: 10.1016/j.bmc.2012.01.014
BindingDB Entry DOI: 10.7270/Q26T0N3Z |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50296782

((1R,2S,3S,5S)-methyl 8-((E)-3-iodoallyl)-3-p-tolyl...)Show SMILES COC(=O)[C@@H]1[C@H]2CC[C@@H](C[C@@H]1c1ccc(C)cc1)N2C\C=C\I |r,THB:19:18:4.10.9:6.7| Show InChI InChI=1S/C19H24INO2/c1-13-4-6-14(7-5-13)16-12-15-8-9-17(18(16)19(22)23-2)21(15)11-3-10-20/h3-7,10,15-18H,8-9,11-12H2,1-2H3/b10-3+/t15-,16+,17+,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Vienna
Curated by ChEMBL
| Assay Description
Binding affinity to NET (unknown origin) |
Bioorg Med Chem 21: 7562-9 (2013)
Article DOI: 10.1016/j.bmc.2013.10.046
BindingDB Entry DOI: 10.7270/Q27P92BD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data