

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM264112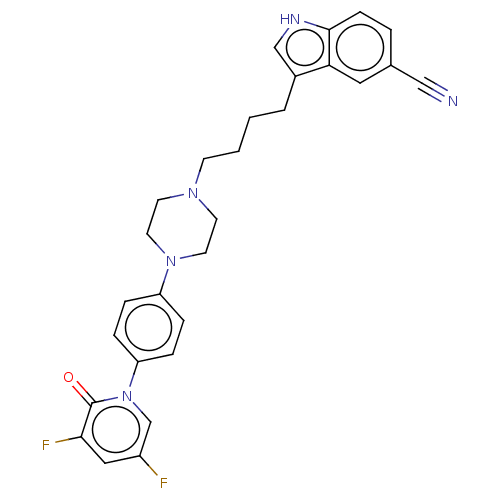 (3-(4-(4-(4-(3,5-difluoro-2-oxopyridin-1(2H)-yl)phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description Human HEK-293 cell homogenates (36 μg protein) were incubated at 22° C. for 60 minutes with 0.3 nM [3H]8-0H-DPAT (Perkin-Elmer) in the absence o... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM264112 (3-(4-(4-(4-(3,5-difluoro-2-oxopyridin-1(2H)-yl)phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
SUNSHINE LAKE PHARMA CO., LTD. US Patent | Assay Description The synaptosomes (150 μg) prepared from a rat brain were incubated at 37° C. for 15 minutes with 0.1 μCi [3H]5-HT in the absence or presenc... | US Patent US9714232 (2017) BindingDB Entry DOI: 10.7270/Q28054N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||