

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044616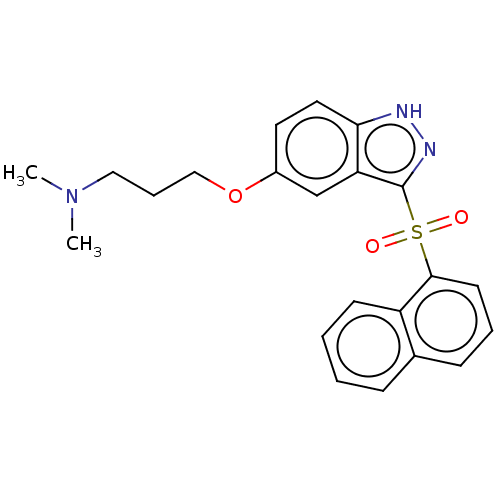 (Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense de Madrid Curated by ChEMBL | Assay Description Antagonist activity at human recombinant 5HT6 receptor expressed in CHO cells | J Med Chem 57: 7160-81 (2014) Article DOI: 10.1021/jm5003952 BindingDB Entry DOI: 10.7270/Q2X92CZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044616 (Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128275 BindingDB Entry DOI: 10.7270/Q2VQ36SJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50044616 (Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Effective concentration required for agonistic activity at Metabotropic glutamate receptor 2 | J Med Chem 60: 1843-1859 (2017) Article DOI: 10.1021/acs.jmedchem.6b01662 BindingDB Entry DOI: 10.7270/Q27S7R1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||