

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828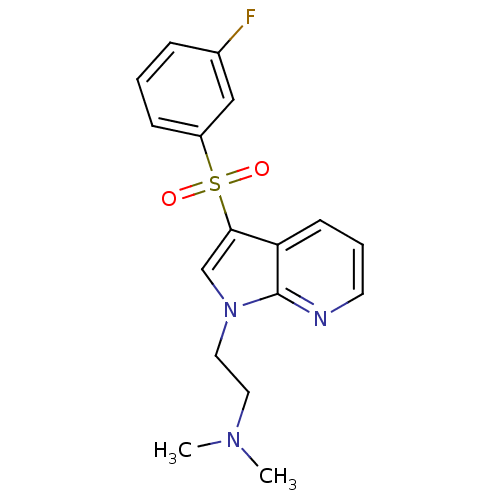 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Displacement of [3H]lysergic acid diethylamide from human 5-HT6 receptor expressed in HeLa cells by scintillation counter | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128275 BindingDB Entry DOI: 10.7270/Q2VQ36SJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human HT6 receptor expressed in human HeLa cells | Bioorg Med Chem Lett 19: 6935-8 (2009) Article DOI: 10.1016/j.bmcl.2009.10.067 BindingDB Entry DOI: 10.7270/Q20K28PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity at human cloned 5HT6 receptor expressed in HeLa cells assessed as induction of adenylyl cyclase activity by RIA | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
Universit£ de Caen Basse-Normandie Curated by ChEMBL | Assay Description Agonist activity at human 5-HT6 receptor expressed in HeLa cells after 10 mins by scintillation proximity assay | J Med Chem 58: 7901-12 (2015) Article DOI: 10.1021/acs.jmedchem.5b00179 BindingDB Entry DOI: 10.7270/Q2M90BGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300828 (2-(3-(3-fluorophenylsulfonyl)-1H-pyrrolo[2,3-b]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity at human HT6 receptor expressed in human HeLa cells assessed as intracellular cAMP level by radioimmunoassay | Bioorg Med Chem Lett 19: 6935-8 (2009) Article DOI: 10.1016/j.bmcl.2009.10.067 BindingDB Entry DOI: 10.7270/Q20K28PH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||