 Found 10 hits Enz. Inhib. hit(s) with Target = 'Beta-1 adrenergic receptor' and Ligand = 'BDBM25753'
Found 10 hits Enz. Inhib. hit(s) with Target = 'Beta-1 adrenergic receptor' and Ligand = 'BDBM25753' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25753
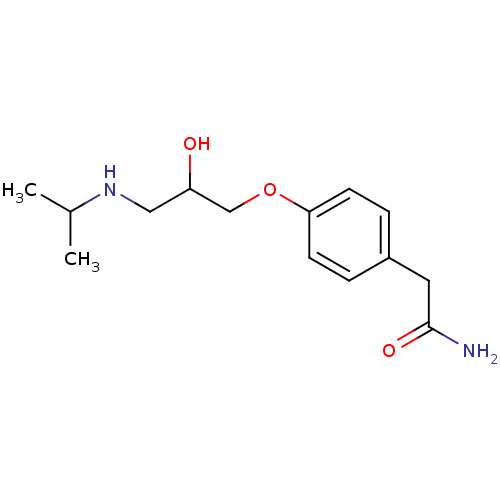
(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 97.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Niigata College of Pharmacy
Curated by PDSP Ki Database
| |
Jpn J Pharmacol 52: 195-200 (1990)
Article DOI: 10.1254/jjp.52.195
BindingDB Entry DOI: 10.7270/Q2H41PXD |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-CGP12177 from human adrenergic beta1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Würzburg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 369: 151-9 (2004)
Article DOI: 10.1007/s00210-003-0860-y
BindingDB Entry DOI: 10.7270/Q2PZ57C6 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGP12177 from human beta1 ADR expressed in HEK293T cell membranes after 90 mins by scintillation counting |
J Med Chem 61: 5380-5394 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00625
BindingDB Entry DOI: 10.7270/Q2XS5XX3 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA |
J Med Chem 48: 6887-96 (2005)
Article DOI: 10.1021/jm058018d
BindingDB Entry DOI: 10.7270/Q28C9X1X |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)CGP12177 from human recombinant Beta-1 adrenergic receptor expressed in HEK293 cells |
Bioorg Med Chem 24: 1793-810 (2016)
Article DOI: 10.1016/j.bmc.2016.03.006
BindingDB Entry DOI: 10.7270/Q2J67JS7 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP 12177 from human recombinant beta1 adrenergic receptor expressed in HEK293 cells measured after 60 mins by scintillation coun... |
Bioorg Med Chem 25: 471-482 (2017)
Article DOI: 10.1016/j.bmc.2016.11.014
BindingDB Entry DOI: 10.7270/Q2CF9S3S |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human alpha1 adrenoceptor |
Bioorg Med Chem 18: 7675-99 (2010)
Article DOI: 10.1016/j.bmc.2010.07.034
BindingDB Entry DOI: 10.7270/Q2DF6S69 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 219 | n/a | n/a | n/a | 7.4 | 37 |
University of Nottingham
| Assay Description
The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined... |
Br J Pharmacol 144: 317-22 (2005)
Article DOI: 10.1038/sj.bjp.0706048
BindingDB Entry DOI: 10.7270/Q28C9TKV |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(GUINEA PIG) | BDBM25753

(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro beta-1 adrenergic receptor activity was determined via inhibition of the positive chronotropic actions of isoproterenol in isolated guinea p... |
J Med Chem 26: 950-7 (1983)
BindingDB Entry DOI: 10.7270/Q2GH9K4C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data