

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971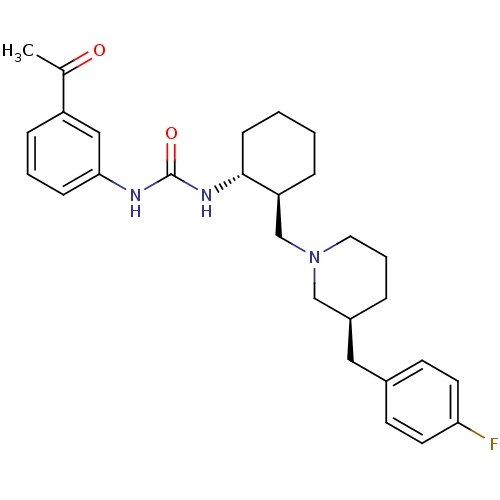 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as eotaxin-induced chemotaxis in human eosinophils | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 assessed as inhibition of chemotaxis by cell based assay | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 receptor in human eosinophil assessed as inhibition of eotaxin-induced chemotaxis | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of eotaxin-induced chemotaxis of Cynomolgus monkey eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]eotaxin from human CCR3 expressed in CHO cells after 30 mins | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]eotaxin from human CCR3 receptor expressed in CHO cells | Bioorg Med Chem Lett 18: 576-85 (2008) Article DOI: 10.1016/j.bmcl.2007.11.067 BindingDB Entry DOI: 10.7270/Q2057GS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Heart and Lung Institute Curated by ChEMBL | Assay Description Binding affinity to CCR3 | J Med Chem 55: 9363-92 (2012) Article DOI: 10.1021/jm300682j BindingDB Entry DOI: 10.7270/Q2862HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in human eosinophils assessed as eotaxin-induced calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 17: 2992-7 (2007) Article DOI: 10.1016/j.bmcl.2007.03.065 BindingDB Entry DOI: 10.7270/Q2V987SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50209971 (1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of calcium mobilization in human eosinophils | J Med Chem 48: 2194-211 (2005) Article DOI: 10.1021/jm049530m BindingDB Entry DOI: 10.7270/Q2XP74F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||