

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50335938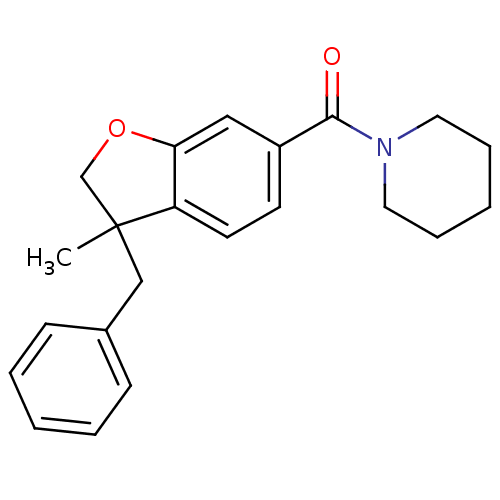 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from rat CB2 receptor expressed in CHO-K1 cells by liquid scintillation spectrophotometry | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO-K1 cells by liquid scintillation counting | Cell Chem Biol 56: 8224-56 (2013) Article DOI: 10.1021/jm4005626 BindingDB Entry DOI: 10.7270/Q2B859M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | 422 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Cleveland Clinic Foundation US Patent | Assay Description Cell membrane homogenates (15 μg protein) were incubated for 120 min at 37° C. with 0.8 nM [3H]WIN 55212-2 (the reference standard [Munro, 1993 ... | US Patent US9339486 (2016) BindingDB Entry DOI: 10.7270/Q2DN43W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 422 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor in cell free system | Eur J Med Chem 46: 547-55 (2011) Article DOI: 10.1016/j.ejmech.2010.11.034 BindingDB Entry DOI: 10.7270/Q2CF9QCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | n/a | n/a | 128 | n/a | n/a | n/a | n/a |
The Cleveland Clinic Foundation US Patent | Assay Description Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma... | US Patent US9339486 (2016) BindingDB Entry DOI: 10.7270/Q2DN43W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
The Cleveland Clinic Foundation US Patent | Assay Description Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma... | US Patent US9339486 (2016) BindingDB Entry DOI: 10.7270/Q2DN43W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50335938 ((3-benzyl-3-methyl-2,3-dihydrobenzofuran-6-yl)(pip...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | n/a | n/a | 21.7 | n/a | n/a | n/a | n/a |
The Cleveland Clinic Foundation US Patent | Assay Description The compound of Example 3 was tested for agonist activity at the rat CB1 (rCB1) and rCB2 receptors, at eight concentrations, in duplicate: 10, 3, 1, ... | US Patent US9339486 (2016) BindingDB Entry DOI: 10.7270/Q2DN43W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||