

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736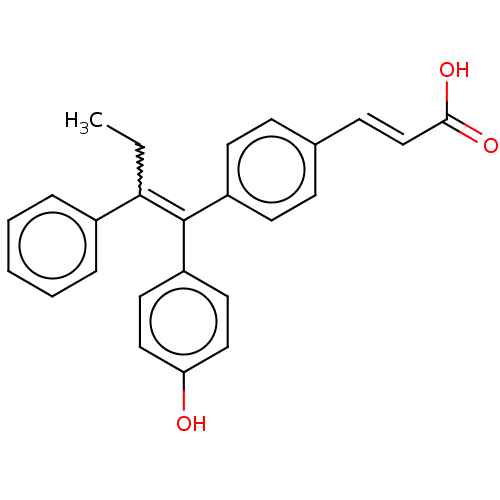 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from full-length human ERalpha receptor by scintillation counting | J Med Chem 60: 6321-6336 (2017) Article DOI: 10.1021/acs.jmedchem.7b00585 BindingDB Entry DOI: 10.7270/Q2RB76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Displacement of fluorescein-labeled estradiol (fluoromone) from human recombinant full-length estrogen receptor alpha after 2 hrs by fluorescence pol... | J Med Chem 61: 514-534 (2018) Article DOI: 10.1021/acs.jmedchem.6b01917 BindingDB Entry DOI: 10.7270/Q20K2C6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of fluorescent-labeled E2 from LBD of ERalpha (unknown origin) by TR-FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02230 BindingDB Entry DOI: 10.7270/Q26H4N92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238736 (CHEMBL1091535 | GW7604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at ERalpha (unknown origin) expressed in human U2OS cells assessed as inhibition of E2-induced transactivation by dual luciferase... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02230 BindingDB Entry DOI: 10.7270/Q26H4N92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||