

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104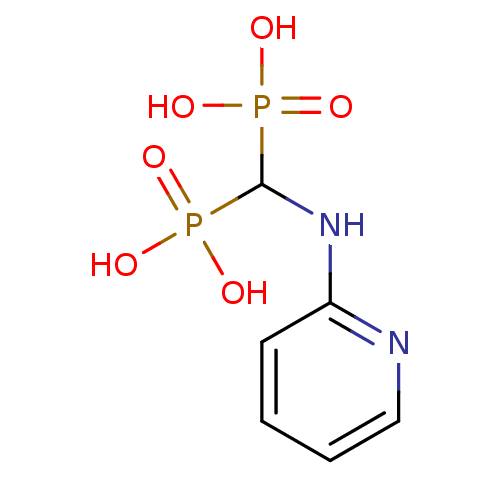 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins | J Med Chem 51: 2187-95 (2008) Article DOI: 10.1021/jm7015733 BindingDB Entry DOI: 10.7270/Q2W95B18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 | J Med Chem 51: 2187-95 (2008) Article DOI: 10.1021/jm7015733 BindingDB Entry DOI: 10.7270/Q2W95B18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins | J Med Chem 51: 2187-95 (2008) Article DOI: 10.1021/jm7015733 BindingDB Entry DOI: 10.7270/Q2W95B18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... | J Med Chem 55: 3201-15 (2012) Article DOI: 10.1021/jm201657x BindingDB Entry DOI: 10.7270/Q29024V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 | J Med Chem 51: 2187-95 (2008) Article DOI: 10.1021/jm7015733 BindingDB Entry DOI: 10.7270/Q2W95B18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M) | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibitory activity against farnesyl Pyrophosphate Synthase was determined | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||