

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127845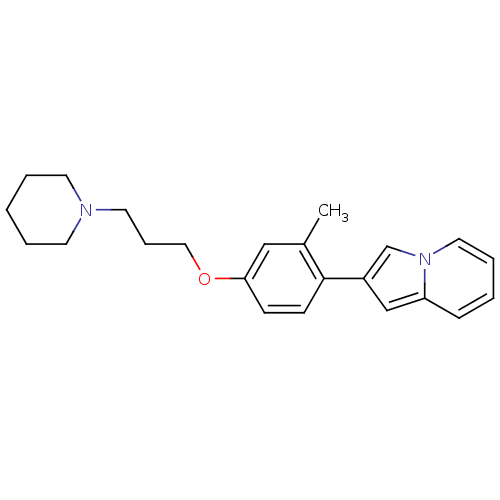 (2-[2-Methyl-4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Botswana Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor | Eur J Med Chem 46: 5237-57 (2011) Article DOI: 10.1016/j.ejmech.2011.08.042 BindingDB Entry DOI: 10.7270/Q2ZG6SNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127845 (2-[2-Methyl-4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||