

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049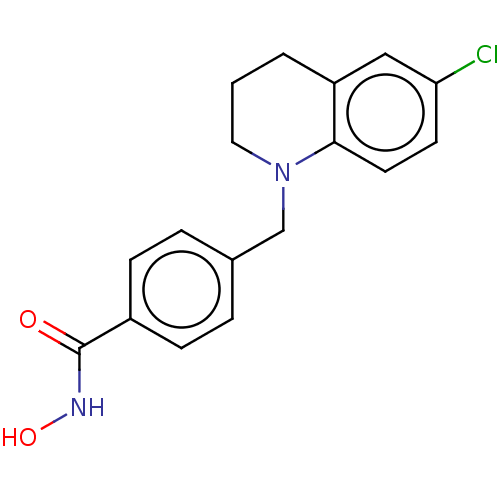 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 insect cells using RHKKAc fluorogenic peptide as substrate pr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02210 BindingDB Entry DOI: 10.7270/Q2222ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Nanoluc-fused HDAC1 (unknown origin) expressed in HEK293 cells incubated for 2 hrs by NANOBRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02210 BindingDB Entry DOI: 10.7270/Q2222ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 769 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human HDAC1 using Ac-GAK(Ac)-AMC as substrate preincubated for 10 mins followed by substrate addition and measured after 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02210 BindingDB Entry DOI: 10.7270/Q2222ZJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human full length recombinant HDAC1 using p53 (379 to 382 residues) (RHKK(Ac)AMC) as substrate preincubated for 5 to 10 mins followed b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00830 BindingDB Entry DOI: 10.7270/Q2F76H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description The effectiveness, or potency, of a present HDACI with respect to inhibiting the activity of an HDAC is measured by an IC50 value. The quantitative I... | US Patent US10456394 (2019) BindingDB Entry DOI: 10.7270/Q2Q81GFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 5.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
THE BOARD OF TRUSTEES OF THE UNIVERSITY OF ILLINOIS US Patent | Assay Description The effectiveness, or potency, of a present HDACI with respect to inhibiting the activity of an HDAC is measured by an IC50 value. The quantitative I... | US Patent US10456394 (2019) BindingDB Entry DOI: 10.7270/Q2Q81GFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of recombinant full length C-terminal His/FLAG tagged human HDAC1 (1 to 482 residues) expressed in baculovirus infected sf9 insect cells u... | J Med Chem 62: 8557-8577 (2019) Article DOI: 10.1021/acs.jmedchem.9b00946 BindingDB Entry DOI: 10.7270/Q2542RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01782 BindingDB Entry DOI: 10.7270/Q2G73JP3 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM417049 (4-((6-chloro-3,4-dihydroquinolin-1(2H)-yl)methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114090 BindingDB Entry DOI: 10.7270/Q2FF3XCC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||