

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128054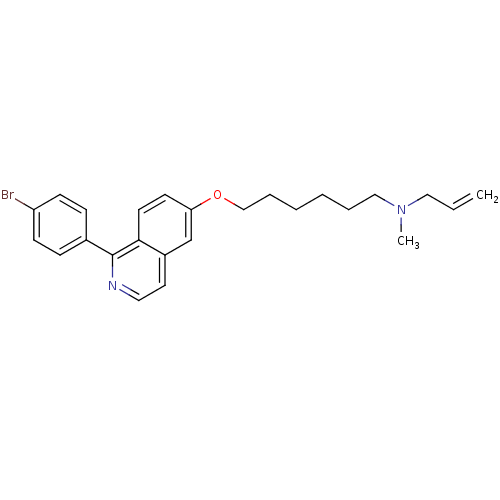 (Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.47 | n/a | n/a | n/a | n/a | n/a | n/a |
CASMedChem Laboratory Curated by ChEMBL | Assay Description Inhibition of oxidosqualene cyclase | Eur J Med Chem 43: 1462-8 (2008) Article DOI: 10.1016/j.ejmech.2007.09.019 BindingDB Entry DOI: 10.7270/Q2CR5VKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128054 (Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Torino Curated by ChEMBL | Assay Description Inhibition of oxidosqualene cyclase | J Med Chem 50: 5039-42 (2007) Article DOI: 10.1021/jm0704651 BindingDB Entry DOI: 10.7270/Q2ZP47FF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128054 (Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-Universität Curated by ChEMBL | Assay Description Inhibitory activity against Oxidosqualene-lanosterol cyclase from human liver microsomes | J Med Chem 46: 2083-92 (2003) Article DOI: 10.1021/jm0211218 BindingDB Entry DOI: 10.7270/Q2VM4BM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128054 (Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of human 2,3-oxidosqualene cyclase. | J Med Chem 46: 3354-70 (2003) Article DOI: 10.1021/jm021120f BindingDB Entry DOI: 10.7270/Q29G5NKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||