

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM263942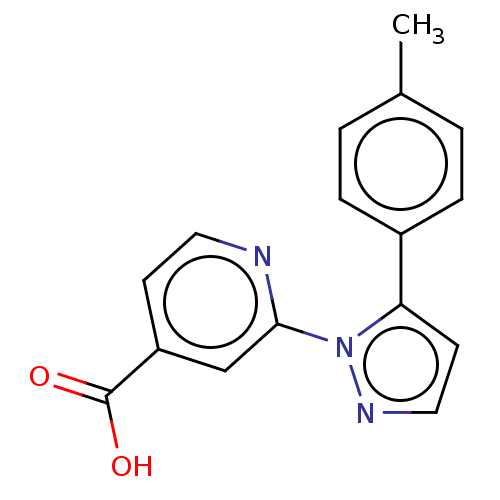 (2-(5-p-tolyl-1H-pyrazol-1- yl)isonicotinic acid | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) preincubated with enzyme | Bioorg Med Chem Lett 28: 1490-1494 (2018) Article DOI: 10.1016/j.bmcl.2018.03.083 BindingDB Entry DOI: 10.7270/Q26Q20W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM263942 (2-(5-p-tolyl-1H-pyrazol-1- yl)isonicotinic acid | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description The ability of test compounds to inhibit the activity of Jarid1B was determined in 384-well plate format under the following reaction conditions: 0.8... | J Med Chem 50: 3359-68 (2007) BindingDB Entry DOI: 10.7270/Q29Z9762 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||