

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346586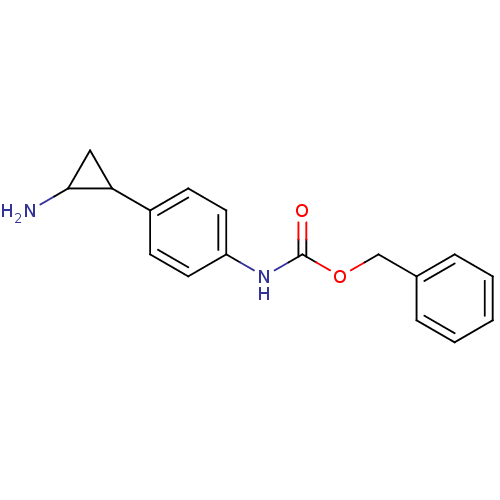 (CHEMBL1795981 | US8765820, 5a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Inhibition of LSD1 | Bioorg Med Chem 19: 3625-36 (2011) Article DOI: 10.1016/j.bmc.2011.01.046 BindingDB Entry DOI: 10.7270/Q23X870S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Universita Degli Studi di Roma “La Sapienza”; Fondazione IEO; Universita Degli Studi di Pavia; Universita Degli Studi di Milano US Patent | Assay Description Human recombinant MAO A and MAO B were expressed in Pichia pastoris and purified as published (Binda C, et al., Proc. Natl. Acad. Sci. USA 100: 9750-... | US Patent US8765820 (2014) BindingDB Entry DOI: 10.7270/Q20P0XPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50346586 (CHEMBL1795981 | US8765820, 5a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton Curated by ChEMBL | Assay Description Inhibition of human LSD1 | Bioorg Med Chem 19: 3709-16 (2011) Article DOI: 10.1016/j.bmc.2011.02.017 BindingDB Entry DOI: 10.7270/Q2J38SWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||