

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445341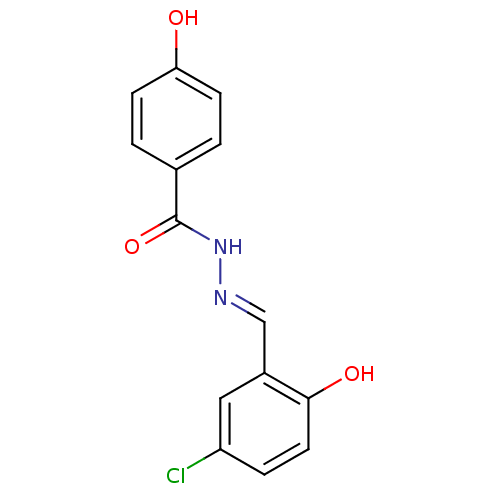 (CHEMBL3104346 | US9555024, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US8987335 (2015) BindingDB Entry DOI: 10.7270/Q2GQ6WGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445341 (CHEMBL3104346 | US9555024, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Research Foundation US Patent | Assay Description The primary assay for compound inhibitory activity was the LSD1 Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, Mich.; Cayman Chem... | US Patent US9555024 (2017) BindingDB Entry DOI: 10.7270/Q21R6SH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445341 (CHEMBL3104346 | US9555024, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of LSD1 (unknown origin) using dimethyl K4 peptide as substrate assessed as resorufin level by spectrophotometric analysis | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||