

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177053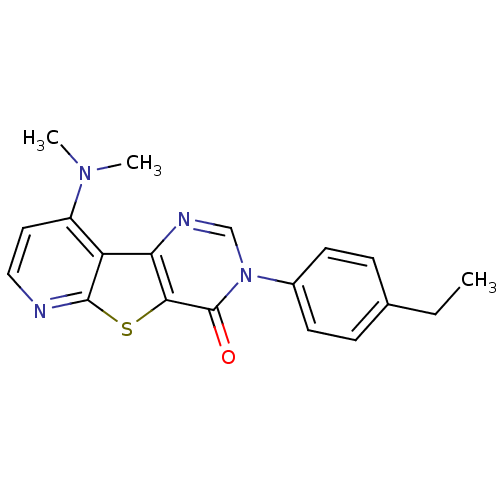 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50177053 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 expressed in 1321N1 cells | Bioorg Med Chem Lett 16: 4936-40 (2006) Article DOI: 10.1016/j.bmcl.2006.06.053 BindingDB Entry DOI: 10.7270/Q2C24W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50177053 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Labs Curated by ChEMBL | Assay Description Antagonist activity at mGlu1 receptor | J Med Chem 50: 2563-8 (2007) Article DOI: 10.1021/jm060950g BindingDB Entry DOI: 10.7270/Q2XS5W7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177053 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocampus Curated by ChEMBL | Assay Description Inhibition of rat mGluR1 | Eur J Med Chem 43: 1025-34 (2008) Article DOI: 10.1016/j.ejmech.2007.06.024 BindingDB Entry DOI: 10.7270/Q2V987VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50177053 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 expressed in 1321N1 cells assessed as effect on L-glutamate-induced calcium mobilization | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177053 (9-Dimethylamino-3-(4-ethyl-phenyl)-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat mGluR1 expressed in 1321N1 cells | Bioorg Med Chem Lett 16: 4936-40 (2006) Article DOI: 10.1016/j.bmcl.2006.06.053 BindingDB Entry DOI: 10.7270/Q2C24W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||