

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50183559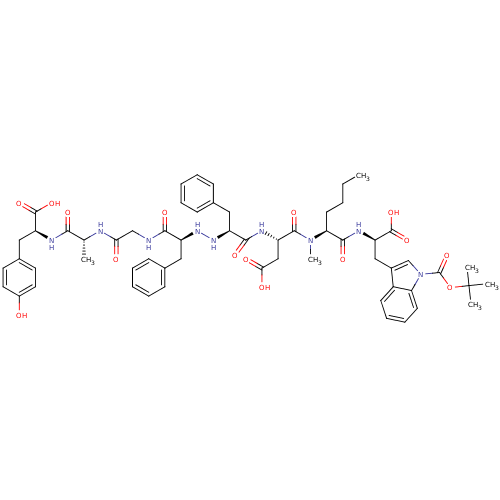 (CHEMBL374325 | Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-NMe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cells | J Med Chem 49: 1773-80 (2006) Article DOI: 10.1021/jm050851n BindingDB Entry DOI: 10.7270/Q2FT8MVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50183559 (CHEMBL374325 | Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-NMe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at mu opioid receptor by inhibition of muscle contraction in electrically stimulated isolated guinea pig ileum at 1 uM | J Med Chem 49: 1773-80 (2006) Article DOI: 10.1021/jm050851n BindingDB Entry DOI: 10.7270/Q2FT8MVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50183559 (CHEMBL374325 | Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-NMe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cells | J Med Chem 49: 1773-80 (2006) Article DOI: 10.1021/jm050851n BindingDB Entry DOI: 10.7270/Q2FT8MVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50183559 (CHEMBL374325 | Tyr-D-Ala-Gly-Phe-NH-NH-Phe-Asp-NMe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S binding to rat mu opioid receptor expressed in CHO cells | J Med Chem 49: 1773-80 (2006) Article DOI: 10.1021/jm050851n BindingDB Entry DOI: 10.7270/Q2FT8MVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||