

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107704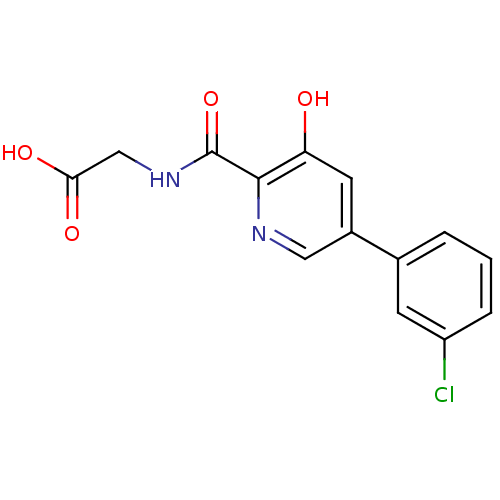 (US11426393, Compound Table XV.11 | US8598210, 119 ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8598210 (2013) BindingDB Entry DOI: 10.7270/Q2M04428 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107704 (US11426393, Compound Table XV.11 | US8598210, 119 ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | 25 |
Akebia Therapeutics, Inc. US Patent | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | US Patent US8722895 (2014) BindingDB Entry DOI: 10.7270/Q25Q4TRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107704 (US11426393, Compound Table XV.11 | US8598210, 119 ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FJ2M1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107704 (US11426393, Compound Table XV.11 | US8598210, 119 ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Los Angeles | Assay Description The EGLN-1 (or EGLN-3) enzyme activity is determined using mass spectrometry (matrix-assisted laser desorption ionization, time-of-flight MS, MALDI-T... | Bioorg Med Chem 17: 7174-85 (2009) BindingDB Entry DOI: 10.7270/Q2D50Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN3 (Homo sapiens (Human)) | BDBM107704 (US11426393, Compound Table XV.11 | US8598210, 119 ...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Akebia Therapeutics, Inc. US Patent | Assay Description HEK293 cells are seeded in 96-well poly-lysine coated plates at 20,000 cells per well in DMEM (10% FBS, 1% NEAA, 0.1% glutamine). Following overnight... | US Patent US9598370 (2017) BindingDB Entry DOI: 10.7270/Q20G3N64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||