

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655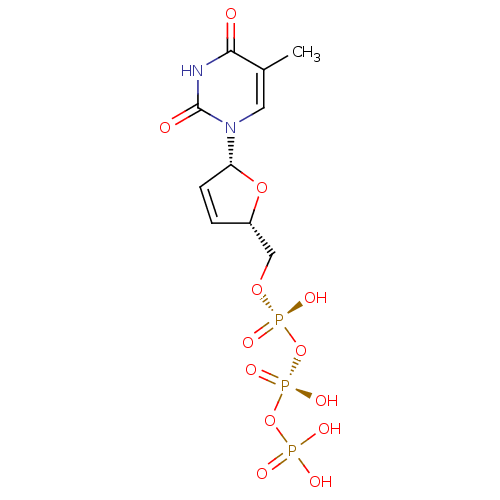 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of HIV1 Reverse transcriptase T165A mutant | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 Reverse transcriptase | Antimicrob Agents Chemother 53: 4640-6 (2009) Article DOI: 10.1128/AAC.00686-09 BindingDB Entry DOI: 10.7270/Q2MC92VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kagoshima University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase | Antimicrob Agents Chemother 51: 2600-4 (2007) Article DOI: 10.1128/aac.00212-07 BindingDB Entry DOI: 10.7270/Q2XW4NKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 15: 5519-28 (2007) Article DOI: 10.1016/j.bmc.2007.05.047 BindingDB Entry DOI: 10.7270/Q20P12S1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Competitive inhibition of C-Terminal histidine-tagged wild type heterodimeric HIV-1 reverse transcriptase p66/p51 assessed as inhibition of dTTP inco... | J Med Chem 56: 3959-68 (2013) Article DOI: 10.1021/jm400160s BindingDB Entry DOI: 10.7270/Q26Q2154 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of DNA-dependent DNA polymerase activity of HIV 1 reverse transcriptase assessed as incorporation of d4TMP by burst assay using DNA/DNA Pr... | Antimicrob Agents Chemother 52: 2035-42 (2008) Article DOI: 10.1128/AAC.00083-08 BindingDB Entry DOI: 10.7270/Q2CZ39Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a |
Yale University Curated by ChEMBL | Assay Description Inhibition of RNA-dependent DNA polymerase activity of HIV 1 reverse transcriptase assessed as incorporation of d4TMP by burst assay using RNA/DNA Pr... | Antimicrob Agents Chemother 52: 2035-42 (2008) Article DOI: 10.1128/AAC.00083-08 BindingDB Entry DOI: 10.7270/Q2CZ39Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50370655 (CHEMBL485652) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh School of Medicine Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase | Antimicrob Agents Chemother 53: 3715-9 (2009) Article DOI: 10.1128/AAC.00392-09 BindingDB Entry DOI: 10.7270/Q2KD21R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||