

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 5 (Mus musculus) | BDBM123216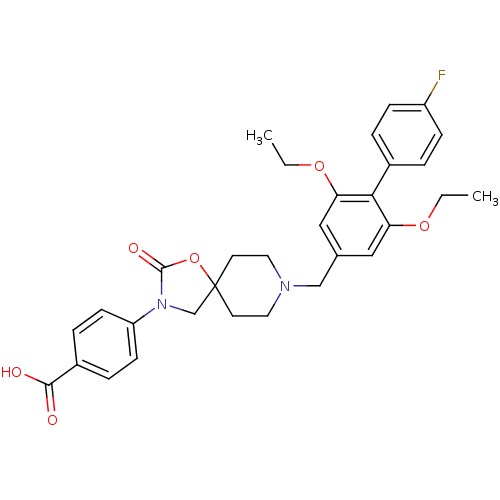 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.768 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||