

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937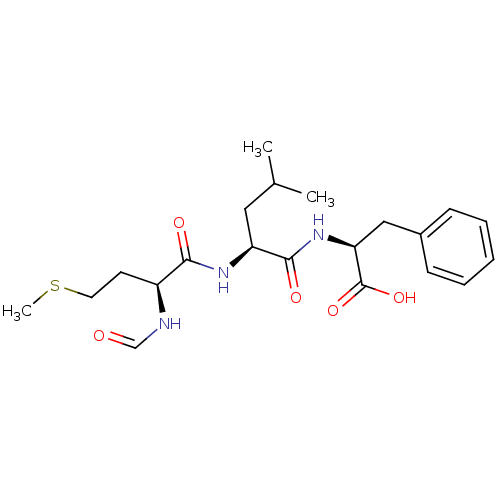 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Displacement of [3H]-fMLP from FPR in human neutrophils | Bioorg Med Chem Lett 17: 3696-701 (2007) Article DOI: 10.1016/j.bmcl.2007.04.036 BindingDB Entry DOI: 10.7270/Q2R21261 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards fMLF receptor using human neutrophils | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Sorbonne Universit£s Curated by ChEMBL | Assay Description Binding affinity to human FPR1 receptor | J Med Chem 58: 1089-99 (2015) Article DOI: 10.1021/jm501018q BindingDB Entry DOI: 10.7270/Q2CN75KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Johnson Matthey Biomedical Research Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring the ability to induce superoxide production(as measured by reduction of cytochrome C) using human neut... | J Med Chem 39: 1013-5 (1996) Article DOI: 10.1021/jm950908d BindingDB Entry DOI: 10.7270/Q2TD9WF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| fMet-Leu-Phe receptor (Homo sapiens (Human)) | BDBM50050937 ((S)-2-[(S)-2-((S)-2-Formylamino-4-methylsulfanyl-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze Curated by ChEMBL | Assay Description Agonist activity at FPR1 in human neutrophiles assessed as induction of intracellular calcium flux by FLIPR3 calcium assay | J Med Chem 52: 5044-57 (2010) Article DOI: 10.1021/jm900592h BindingDB Entry DOI: 10.7270/Q2WS8T85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||