 Found 106 hits Enz. Inhib. hit(s) with all data for assayid = 2 entry = 8679
Found 106 hits Enz. Inhib. hit(s) with all data for assayid = 2 entry = 8679 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM108454
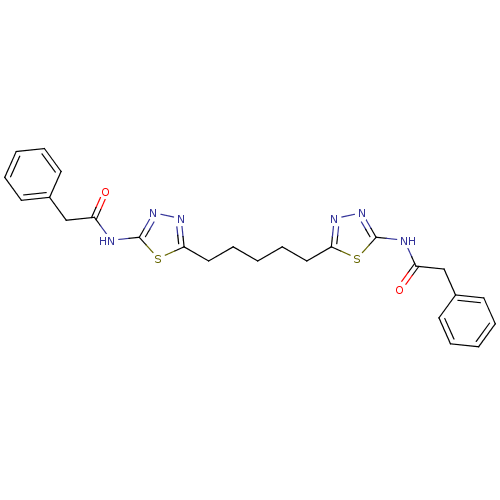
(US8604016, 11 | US9938267, Cmpd ID 11)Show SMILES O=C(Cc1ccccc1)Nc1nnc(CCCCCc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C25H26N6O2S2/c32-20(16-18-10-4-1-5-11-18)26-24-30-28-22(34-24)14-8-3-9-15-23-29-31-25(35-23)27-21(33)17-19-12-6-2-7-13-19/h1-2,4-7,10-13H,3,8-9,14-17H2,(H,26,30,32)(H,27,31,33) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591262
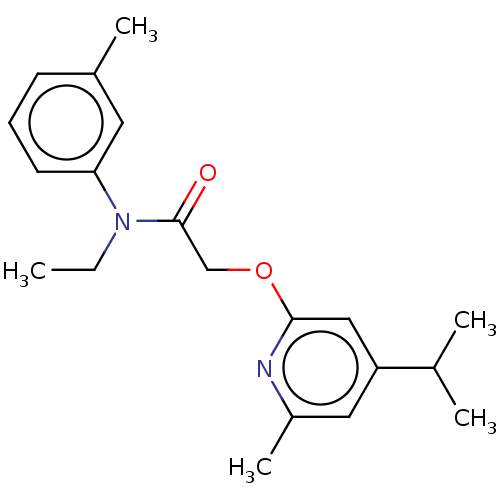
(CHEMBL5186419) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591265
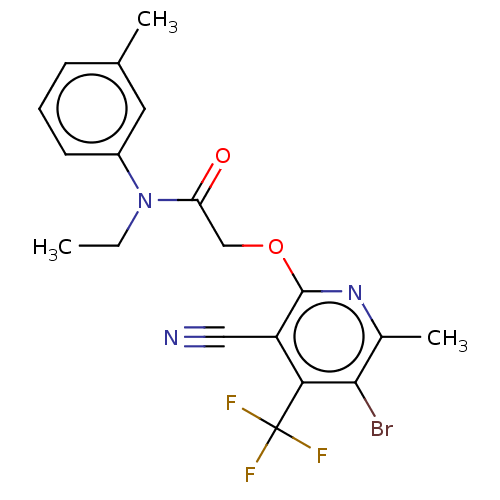
(CHEMBL5188870)Show SMILES CCN(C(=O)COc1nc(C)c(Br)c(c1C#N)C(F)(F)F)c1cccc(C)c1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591268

(CHEMBL5182311)Show SMILES CCN(C(=O)COc1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591263
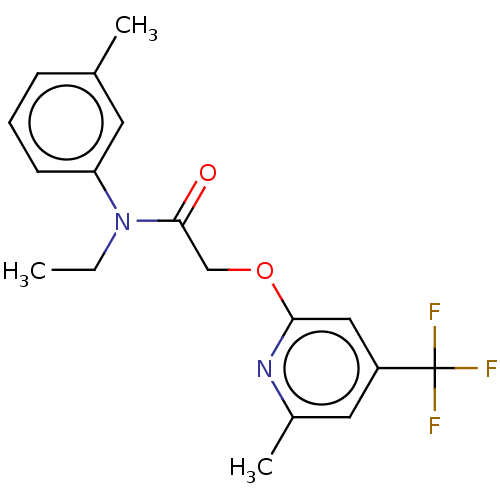
(CHEMBL5180697)Show SMILES CCN(C(=O)COc1cc(cc(C)n1)C(F)(F)F)c1cccc(C)c1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514984
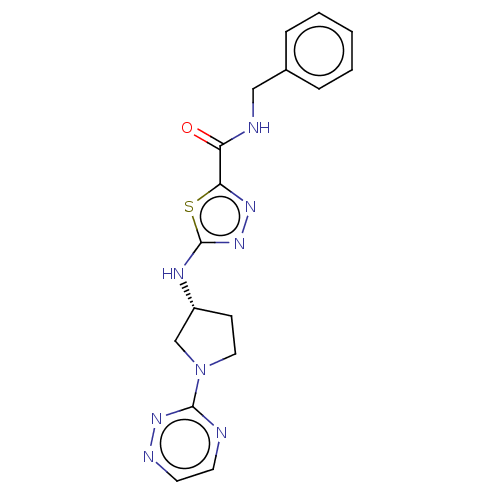
(CHEMBL4534437)Show SMILES O=C(NCc1ccccc1)c1nnc(N[C@@H]2CCN(C2)c2nccnn2)s1 |r| Show InChI InChI=1S/C17H18N8OS/c26-14(19-10-12-4-2-1-3-5-12)15-22-24-17(27-15)21-13-6-9-25(11-13)16-18-7-8-20-23-16/h1-5,7-8,13H,6,9-11H2,(H,19,26)(H,21,24)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591267

(CHEMBL5173309)Show SMILES CCN(C(=O)COc1nc(C)c(Cl)c(C)c1C#N)c1cccc(C)c1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514987

(CHEMBL4570299)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nncs2)s1 |r| Show InChI InChI=1S/C16H17N7OS2/c24-13(8-11-4-2-1-3-5-11)19-15-21-20-14(26-15)18-12-6-7-23(9-12)16-22-17-10-25-16/h1-5,10,12H,6-9H2,(H,18,20)(H,19,21,24)/t12-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 873 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514976

(CHEMBL4458193)Show SMILES Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C16H18N8OS2/c17-13-20-21-14(26-13)18-11-6-7-24(9-11)16-23-22-15(27-16)19-12(25)8-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,17,20)(H,18,21)(H,19,22,25)/t11-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591266
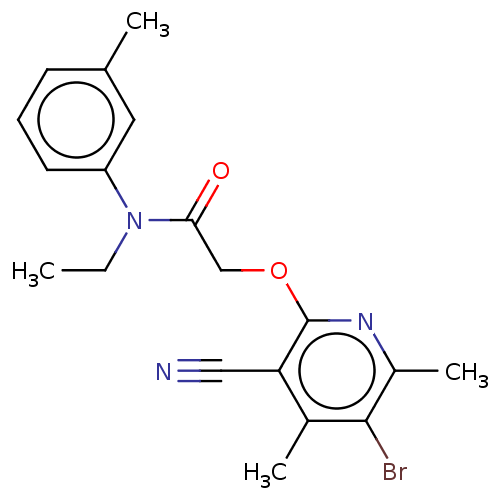
(CHEMBL5196469)Show SMILES CCN(C(=O)COc1nc(C)c(Br)c(C)c1C#N)c1cccc(C)c1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591261
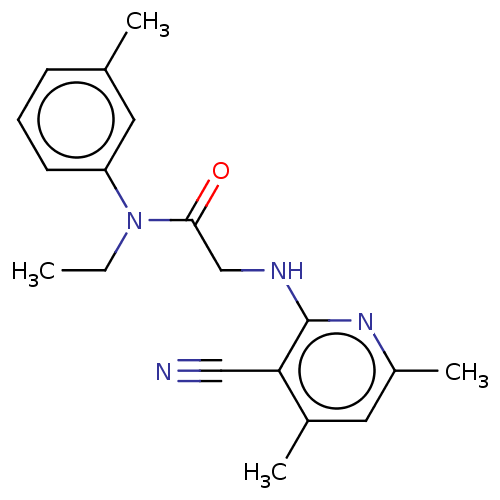
(CHEMBL5192479) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591254
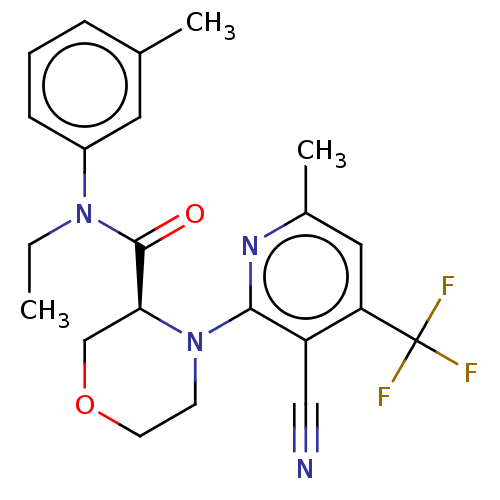
(CHEMBL5177353)Show SMILES CCN(C(=O)[C@@H]1COCCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591264
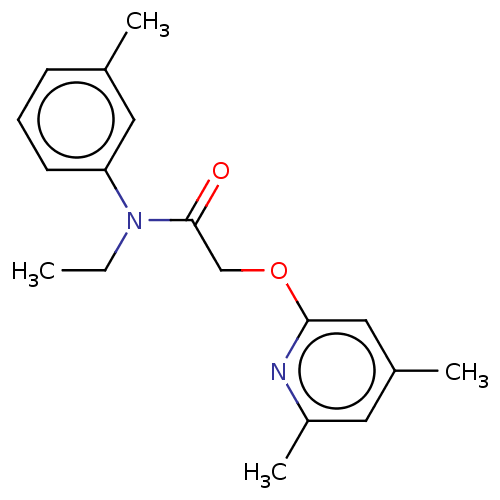
(CHEMBL5204362) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591269
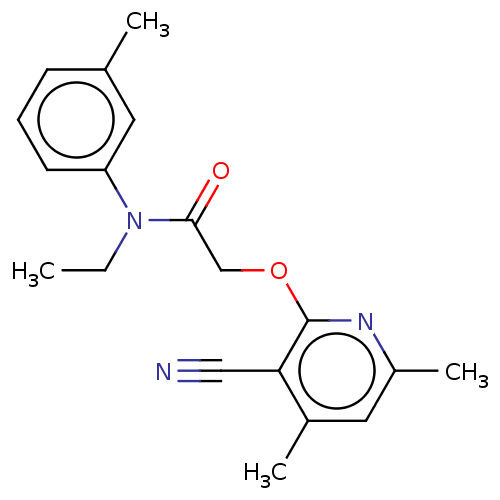
(CHEMBL5172405) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591257

(CHEMBL5173938)Show SMILES CCN(C(=O)[C@@H](C)Nc1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM278400
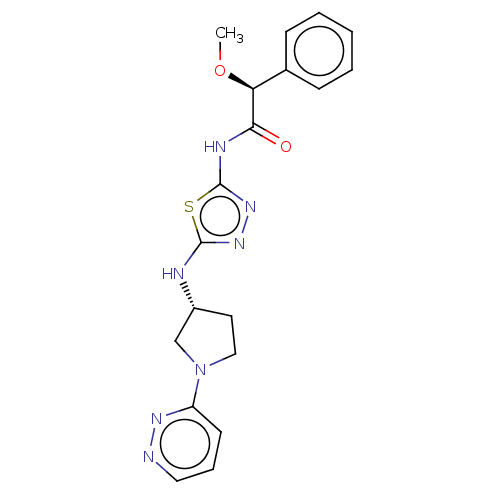
((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NET (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase kidney isoform, mitochondrial
(Homo sapiens (Human)) | BDBM50514981
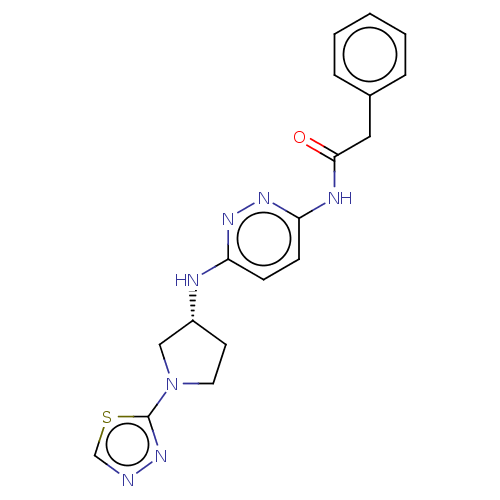
(CHEMBL4434859)Show SMILES O=C(Cc1ccccc1)Nc1ccc(N[C@@H]2CCN(C2)c2nncs2)nn1 |r| Show InChI InChI=1S/C18H19N7OS/c26-17(10-13-4-2-1-3-5-13)21-16-7-6-15(22-23-16)20-14-8-9-25(11-14)18-24-19-12-27-18/h1-7,12,14H,8-11H2,(H,20,22)(H,21,23,26)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant 6His-tagged GLS1 KGA isoform (unknown origin) (63 to 669 residues) expressed in Escherichia coli using glutamine as substra... |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591286

(CHEMBL5198552)Show SMILES CN(C(=O)[C@H]1[C@@H](O)[C@@H](O)C(=O)N1c1cc(cc(C)n1)C(F)(F)F)c1ccc(F)c(Cl)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591248

(CHEMBL5206992)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591250
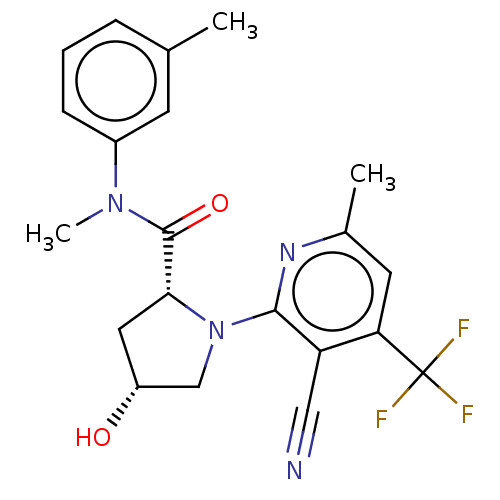
(CHEMBL5169726)Show SMILES CN(C(=O)[C@H]1C[C@@H](O)CN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM278400

((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 5HT2B (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM278400

((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Ionworks electrophysiology assay |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50172138
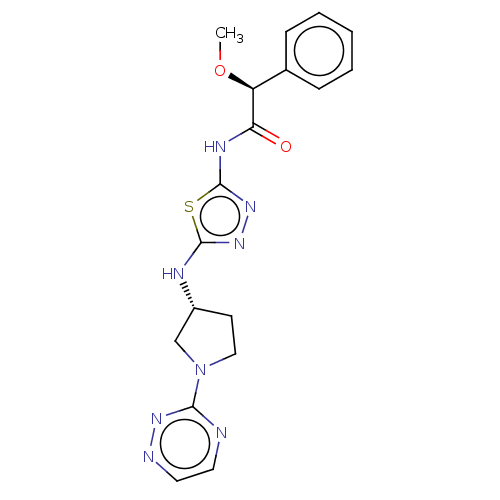
(CHEMBL3808443 | US10040788, Example 1(a) | US10294...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2nccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C18H20N8O2S/c1-28-14(12-5-3-2-4-6-12)15(27)22-18-25-24-17(29-18)21-13-7-10-26(11-13)16-19-8-9-20-23-16/h2-6,8-9,13-14H,7,10-11H2,1H3,(H,21,24)(H,22,25,27)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells by Ionworks electrophysiology assay |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM108464
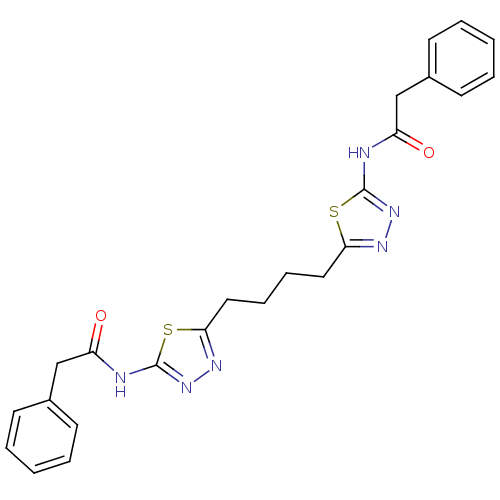
(US8604016, 21 | US9938267, Cmpd ID 21)Show SMILES O=C(Cc1ccccc1)Nc1nnc(CCCCc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C24H24N6O2S2/c31-19(15-17-9-3-1-4-10-17)25-23-29-27-21(33-23)13-7-8-14-22-28-30-24(34-22)26-20(32)16-18-11-5-2-6-12-18/h1-6,9-12H,7-8,13-16H2,(H,25,29,31)(H,26,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514974
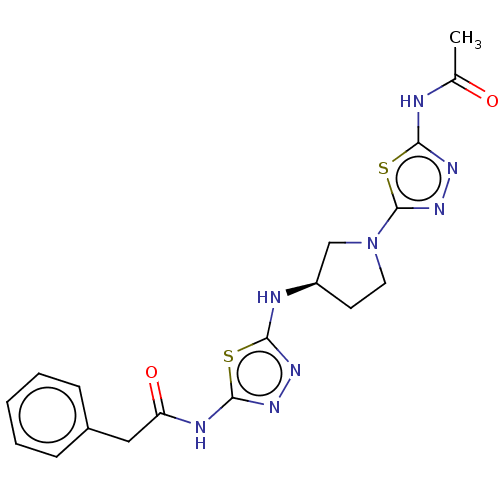
(CHEMBL4472367)Show SMILES CC(=O)Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C18H20N8O2S2/c1-11(27)19-15-24-25-18(30-15)26-8-7-13(10-26)20-16-22-23-17(29-16)21-14(28)9-12-5-3-2-4-6-12/h2-6,13H,7-10H2,1H3,(H,20,22)(H,19,24,27)(H,21,23,28)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50400050
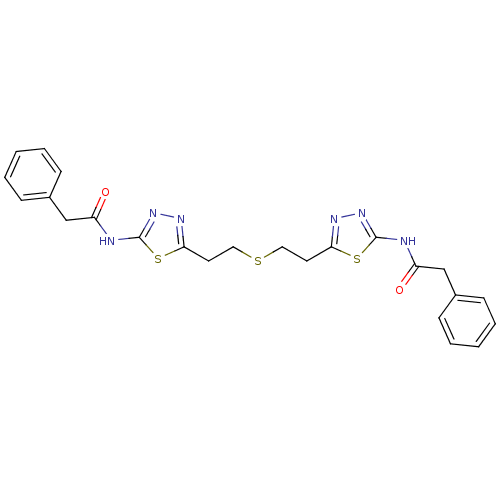
(CHEMBL2177757 | US10793535, Cmpd ID 1 | US11191732...)Show SMILES O=C(Cc1ccccc1)Nc1nnc(CCSCCc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C24H24N6O2S3/c31-19(15-17-7-3-1-4-8-17)25-23-29-27-21(34-23)11-13-33-14-12-22-28-30-24(35-22)26-20(32)16-18-9-5-2-6-10-18/h1-10H,11-16H2,(H,25,29,31)(H,26,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514977
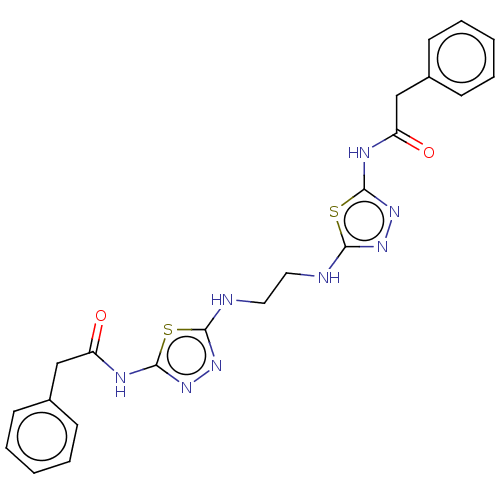
(CHEMBL4461749)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NCCNc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C22H22N8O2S2/c31-17(13-15-7-3-1-4-8-15)25-21-29-27-19(33-21)23-11-12-24-20-28-30-22(34-20)26-18(32)14-16-9-5-2-6-10-16/h1-10H,11-14H2,(H,23,27)(H,24,28)(H,25,29,31)(H,26,30,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514986
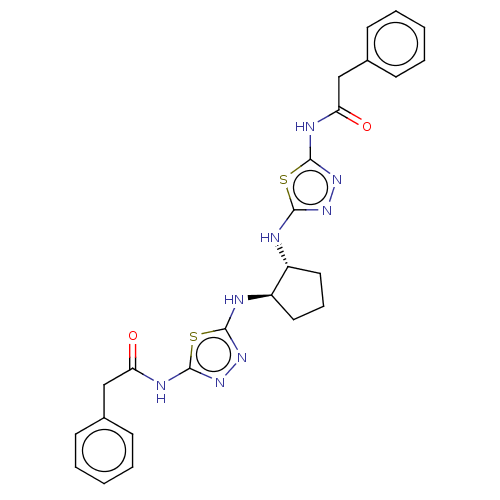
(CHEMBL4437956)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCC[C@H]2Nc2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(14-16-8-3-1-4-9-16)28-24-32-30-22(36-24)26-18-12-7-13-19(18)27-23-31-33-25(37-23)29-21(35)15-17-10-5-2-6-11-17/h1-6,8-11,18-19H,7,12-15H2,(H,26,30)(H,27,31)(H,28,32,34)(H,29,33,35)/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50150109
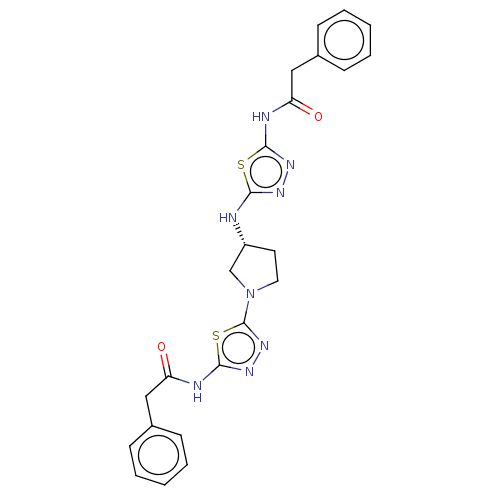
(CHEMBL3770355)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C24H24N8O2S2/c33-19(13-16-7-3-1-4-8-16)26-22-29-28-21(35-22)25-18-11-12-32(15-18)24-31-30-23(36-24)27-20(34)14-17-9-5-2-6-10-17/h1-10,18H,11-15H2,(H,25,28)(H,26,29,33)(H,27,30,34)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514975

(CHEMBL4473143)Show SMILES O=C(Cc1ccccc1)Nc1nnc(NC[C@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C25H26N8O2S2/c34-20(13-17-7-3-1-4-8-17)27-23-30-29-22(36-23)26-15-19-11-12-33(16-19)25-32-31-24(37-25)28-21(35)14-18-9-5-2-6-10-18/h1-10,19H,11-16H2,(H,26,29)(H,27,30,34)(H,28,31,35)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514980

(CHEMBL4438255)Show SMILES Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C16H18N8OS2/c17-13-20-23-16(26-13)24-7-6-11(9-24)18-14-21-22-15(27-14)19-12(25)8-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,17,20)(H,18,21)(H,19,22,25)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514987

(CHEMBL4570299)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nncs2)s1 |r| Show InChI InChI=1S/C16H17N7OS2/c24-13(8-11-4-2-1-3-5-11)19-15-21-20-14(26-15)18-12-6-7-23(9-12)16-22-17-10-25-16/h1-5,10,12H,6-9H2,(H,18,20)(H,19,21,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514982

(CHEMBL4469711)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1 |r| Show InChI InChI=1S/C18H19N7OS/c26-16(11-13-5-2-1-3-6-13)21-18-24-23-17(27-18)20-14-8-10-25(12-14)15-7-4-9-19-22-15/h1-7,9,14H,8,10-12H2,(H,20,23)(H,21,24,26)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514983
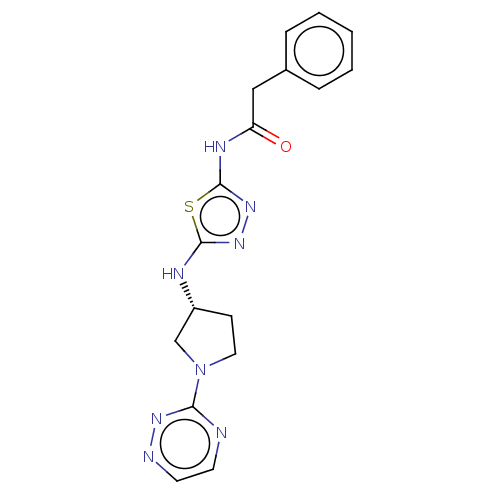
(CHEMBL4447951)Show SMILES O=C(Cc1ccccc1)Nc1nnc(N[C@@H]2CCN(C2)c2nccnn2)s1 |r| Show InChI InChI=1S/C17H18N8OS/c26-14(10-12-4-2-1-3-5-12)21-17-24-23-16(27-17)20-13-6-9-25(11-13)15-18-7-8-19-22-15/h1-5,7-8,13H,6,9-11H2,(H,20,23)(H,21,24,26)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM108512
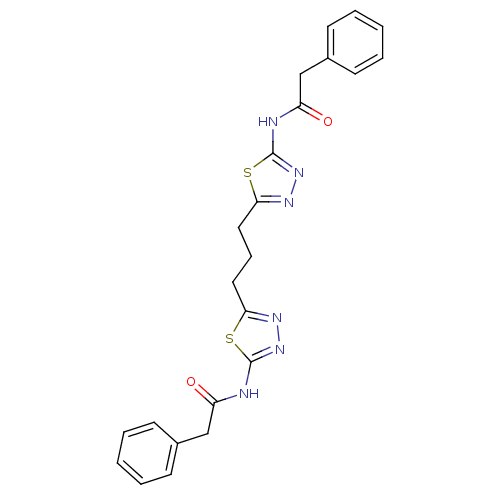
(US8604016, 72 | US9938267, Cmpd ID 72)Show SMILES O=C(Cc1ccccc1)Nc1nnc(CCCc2nnc(NC(=O)Cc3ccccc3)s2)s1 Show InChI InChI=1S/C23H22N6O2S2/c30-18(14-16-8-3-1-4-9-16)24-22-28-26-20(32-22)12-7-13-21-27-29-23(33-21)25-19(31)15-17-10-5-2-6-11-17/h1-6,8-11H,7,12-15H2,(H,24,28,30)(H,25,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514976

(CHEMBL4458193)Show SMILES Nc1nnc(N[C@@H]2CCN(C2)c2nnc(NC(=O)Cc3ccccc3)s2)s1 |r| Show InChI InChI=1S/C16H18N8OS2/c17-13-20-21-14(26-13)18-11-6-7-24(9-11)16-23-22-15(27-16)19-12(25)8-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H2,17,20)(H,18,21)(H,19,22,25)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514978
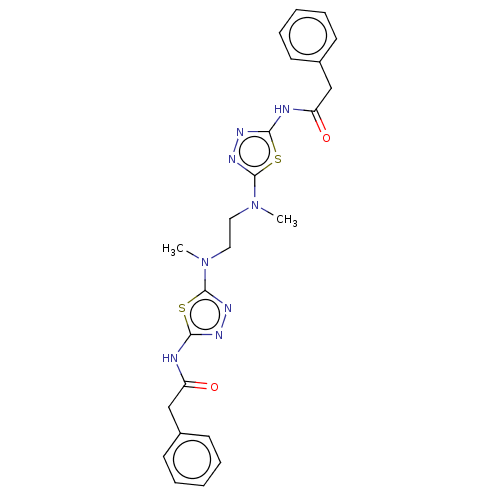
(CHEMBL4451434)Show SMILES CN(CCN(C)c1nnc(NC(=O)Cc2ccccc2)s1)c1nnc(NC(=O)Cc2ccccc2)s1 Show InChI InChI=1S/C24H26N8O2S2/c1-31(23-29-27-21(35-23)25-19(33)15-17-9-5-3-6-10-17)13-14-32(2)24-30-28-22(36-24)26-20(34)16-18-11-7-4-8-12-18/h3-12H,13-16H2,1-2H3,(H,25,27,33)(H,26,28,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514989
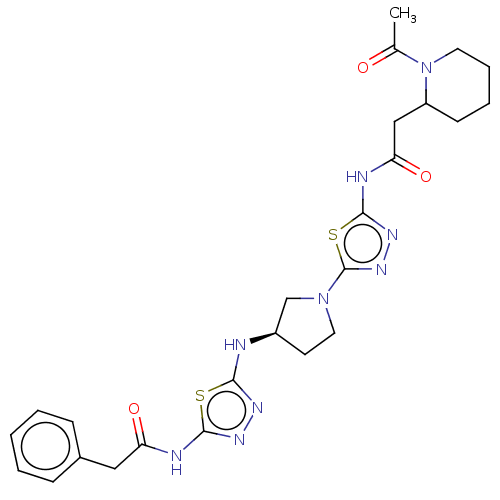
(CHEMBL4444363)Show SMILES CC(=O)N1CCCCC1CC(=O)Nc1nnc(s1)N1CC[C@H](C1)Nc1nnc(NC(=O)Cc2ccccc2)s1 |r| Show InChI InChI=1S/C25H31N9O3S2/c1-16(35)34-11-6-5-9-19(34)14-21(37)28-24-31-32-25(39-24)33-12-10-18(15-33)26-22-29-30-23(38-22)27-20(36)13-17-7-3-2-4-8-17/h2-4,7-8,18-19H,5-6,9-15H2,1H3,(H,26,29)(H,27,30,36)(H,28,31,37)/t18-,19?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Glutaminase liver isoform, mitochondrial
(Homo sapiens) | BDBM50514990

(CHEMBL4448931)Show SMILES Nc1nnc(N[C@@H]2CCN(C2)c2ccc(NC(=O)Cc3ccccc3)nn2)s1 |r| Show InChI InChI=1S/C18H20N8OS/c19-17-24-25-18(28-17)20-13-8-9-26(11-13)15-7-6-14(22-23-15)21-16(27)10-12-4-2-1-3-5-12/h1-7,13H,8-11H2,(H2,19,24)(H,20,25)(H,21,22,27)/t13-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of GLS2 (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM278400

((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of OPRM (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM278400

((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of dopamine transporter (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM278400

((2S)-2-Methoxy-2-phenyl-N-[5-[[(3R)-1-pyridazin-3-...)Show SMILES CO[C@H](C(=O)Nc1nnc(N[C@@H]2CCN(C2)c2cccnn2)s1)c1ccccc1 |r| Show InChI InChI=1S/C19H21N7O2S/c1-28-16(13-6-3-2-4-7-13)17(27)22-19-25-24-18(29-19)21-14-9-11-26(12-14)15-8-5-10-20-23-15/h2-8,10,14,16H,9,11-12H2,1H3,(H,21,24)(H,22,25,27)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of NK1R (unknown origin) |
J Med Chem 62: 6540-6560 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00260
BindingDB Entry DOI: 10.7270/Q2QN6B4W |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591287
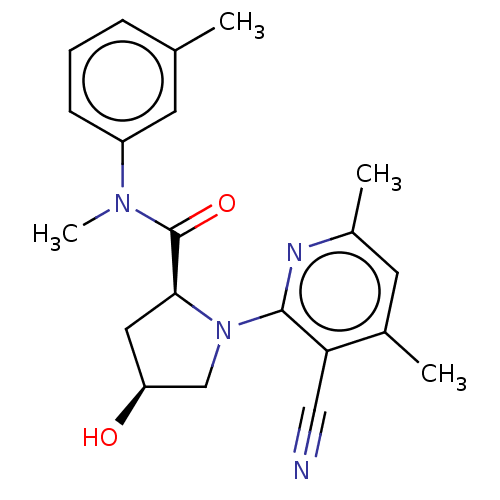
(CHEMBL5186881)Show SMILES CN(C(=O)[C@@H]1C[C@H](O)CN1c1nc(C)cc(C)c1C#N)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591269

(CHEMBL5172405) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591289
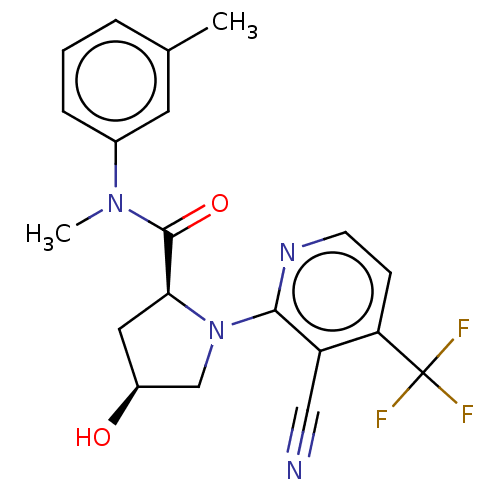
(CHEMBL5169944)Show SMILES CN(C(=O)[C@@H]1C[C@H](O)CN1c1nccc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591253
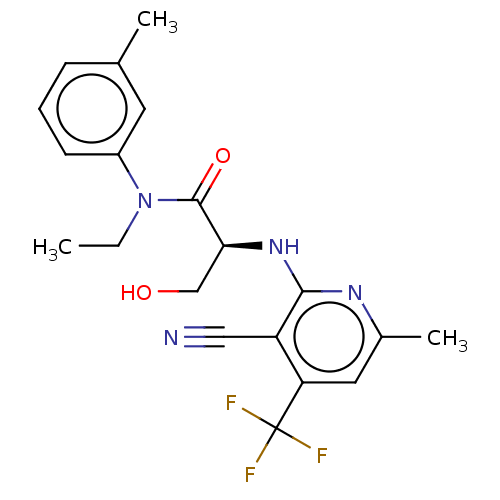
(CHEMBL5174548)Show SMILES CCN(C(=O)[C@H](CO)Nc1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591251
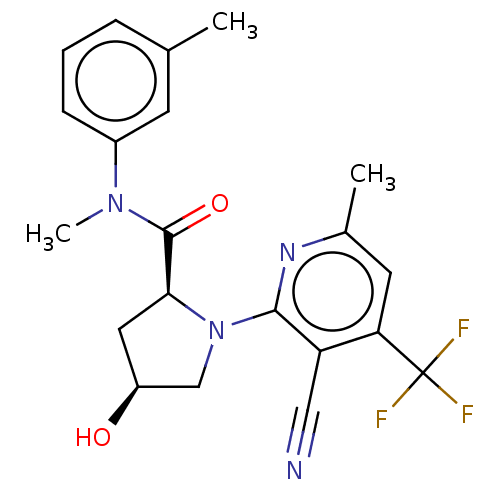
(CHEMBL5206829)Show SMILES CN(C(=O)[C@@H]1C[C@H](O)CN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591249
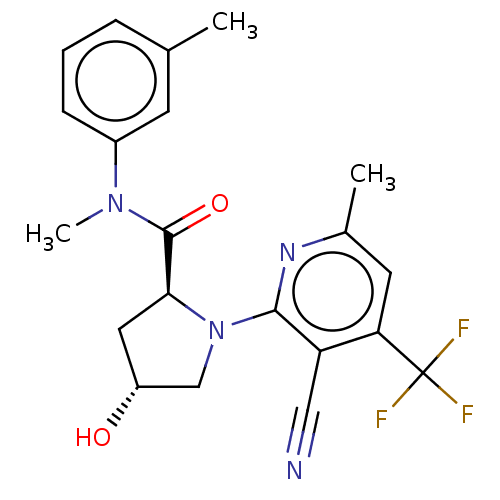
(CHEMBL5170837)Show SMILES CN(C(=O)[C@@H]1C[C@@H](O)CN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591248

(CHEMBL5206992)Show SMILES CN(C(=O)[C@@H]1[C@H](O)CCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591247
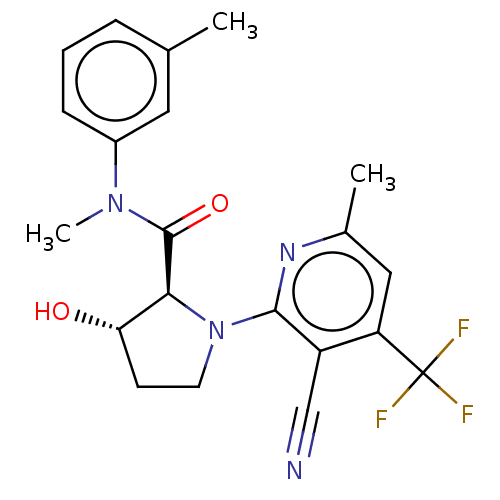
(CHEMBL5206124)Show SMILES CN(C(=O)[C@@H]1[C@@H](O)CCN1c1nc(C)cc(c1C#N)C(F)(F)F)c1cccc(C)c1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01142
BindingDB Entry DOI: 10.7270/Q2Z03D46 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data