

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9022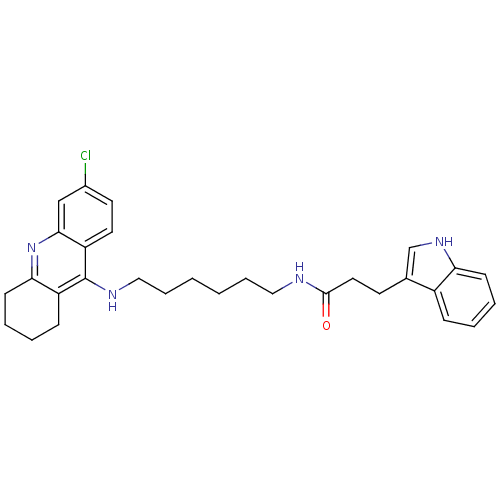 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9027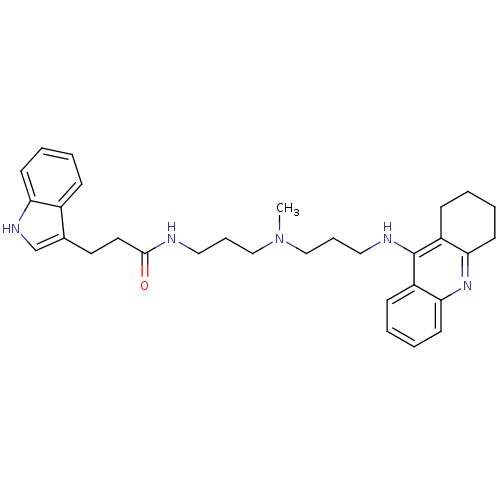 (3-(1H-indol-3-yl)-N-(3-{methyl[3-(1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9023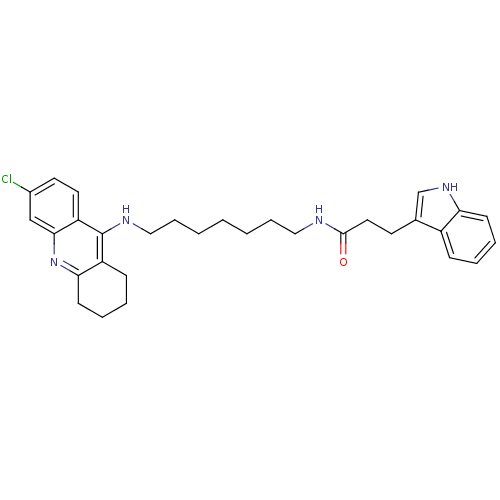 (CHEMBL225198 | Indole-Tacrine Heterodimer 6 | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9023 (CHEMBL225198 | Indole-Tacrine Heterodimer 6 | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9035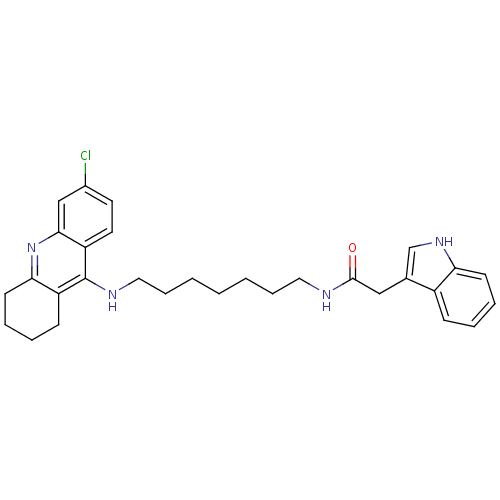 (Indole-Tacrine Heterodimer 18 | N-[7-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9037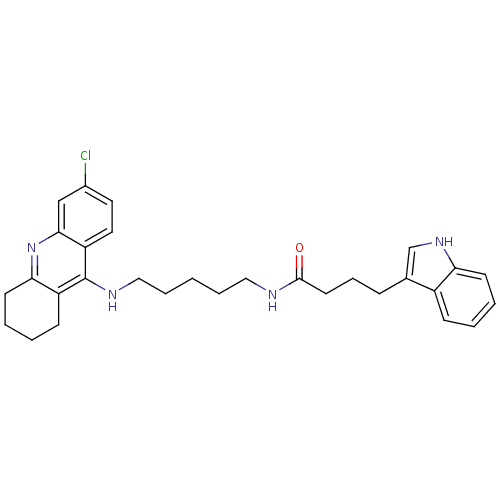 (Indole-Tacrine Heterodimer 20 | N-[5-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9024 (Indole-Tacrine Heterodimer 7 | N-[8-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9038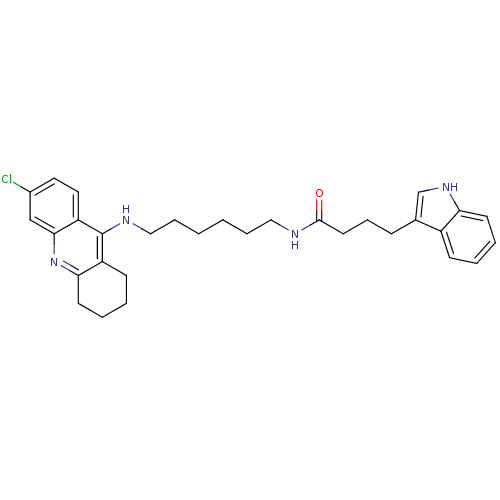 (Indole-Tacrine Heterodimer 21 | N-[6-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9036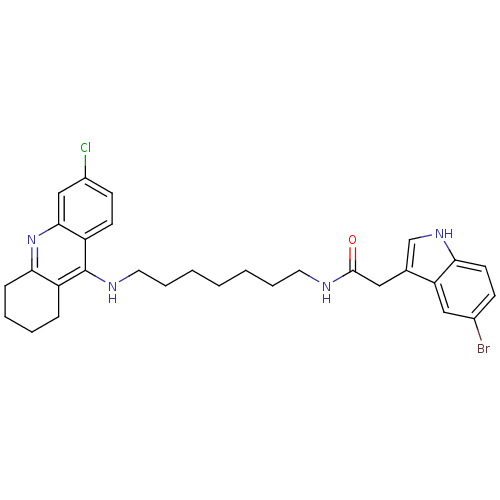 (2-(5-Bromo-1H-indol-3-yl)-N-[7-(6-chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9063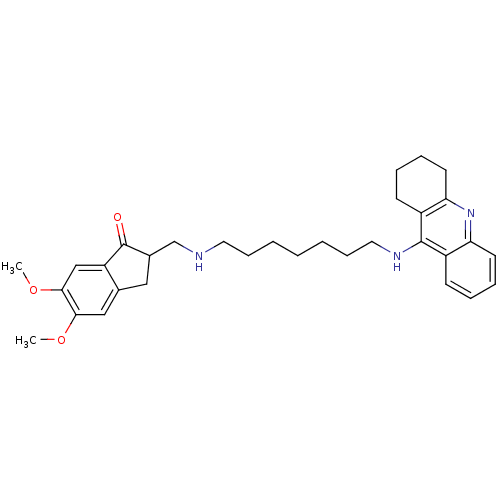 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9042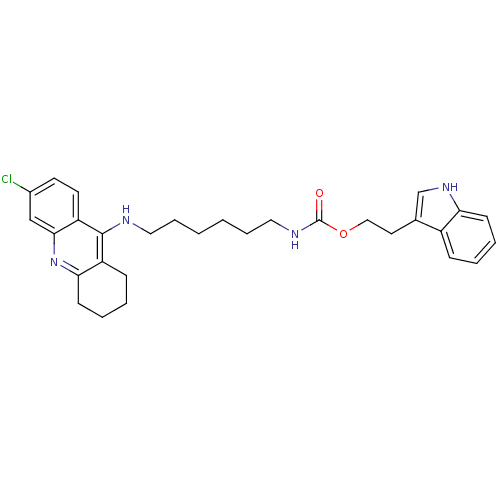 (2-(1H-indol-3-yl)ethyl N-{6-[(6-chloro-1,2,3,4-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9029 (Indole-Tacrine Heterodimer 12 | N-[6-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9020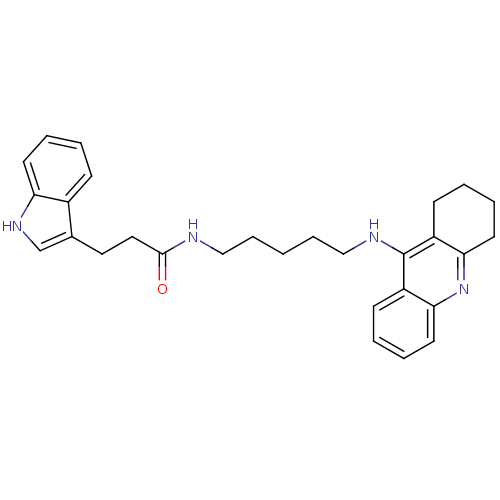 (3-(1H-Indol-3-yl)-N-[5-(1,2,3,4-tetrahydroacridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9041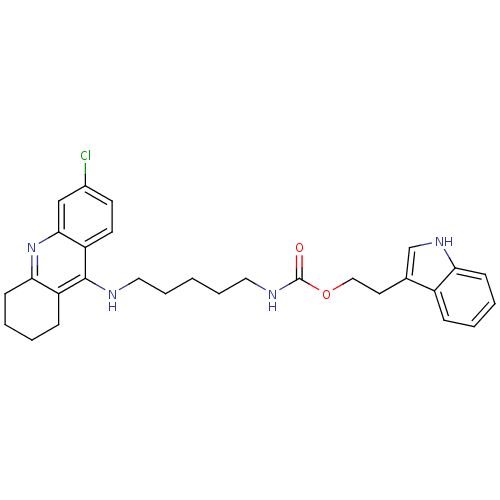 (2-(1H-indol-3-yl)ethyl N-{5-[(6-chloro-1,2,3,4-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9032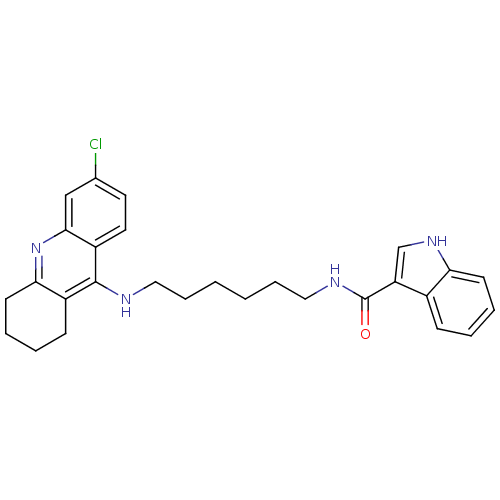 (1H-Indole-3-carboxylic Acid [6-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9036 (2-(5-Bromo-1H-indol-3-yl)-N-[7-(6-chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9073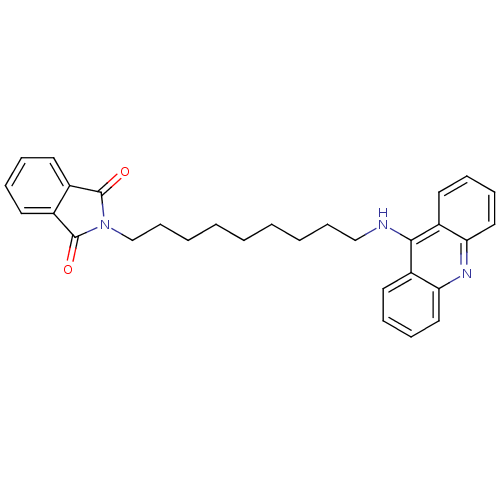 (2-[9-(Acridin-9-ylamino)-nonyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9067 (Donepezil-tacrine hybrid 12 | N-[4-({2-[(6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9028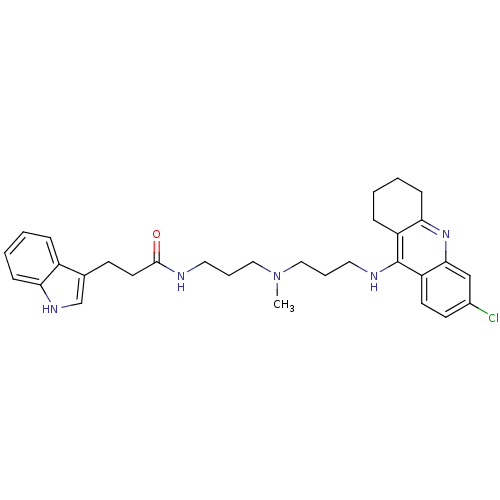 (Indole-Tacrine Heterodimer 11 | N-(3-{[3-(6-Chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9022 (CHEMBL225567 | Indole-Tacrine Heterodimer 5 | N-[5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9043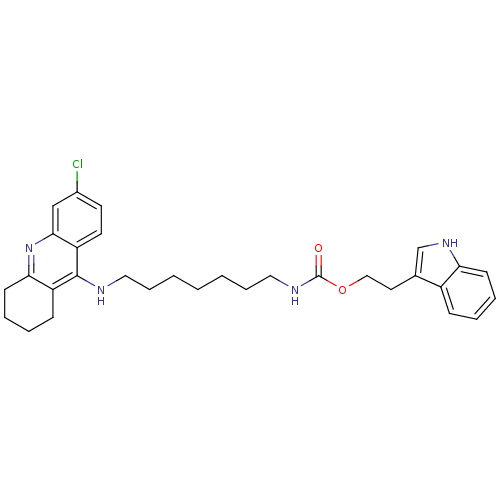 (2-(1H-indol-3-yl)ethyl N-{7-[(6-chloro-1,2,3,4-tet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9037 (Indole-Tacrine Heterodimer 20 | N-[5-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9021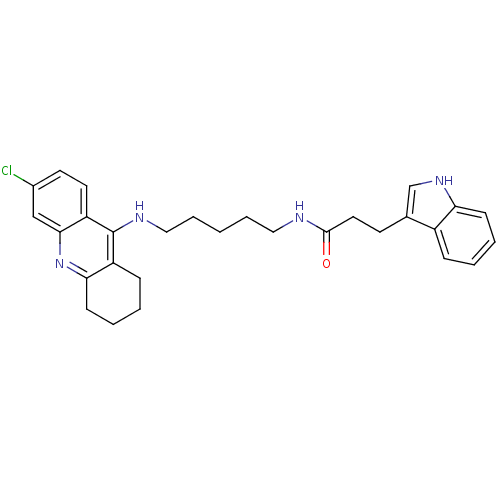 (Indole-Tacrine Heterodimer 4 | N-[5-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9025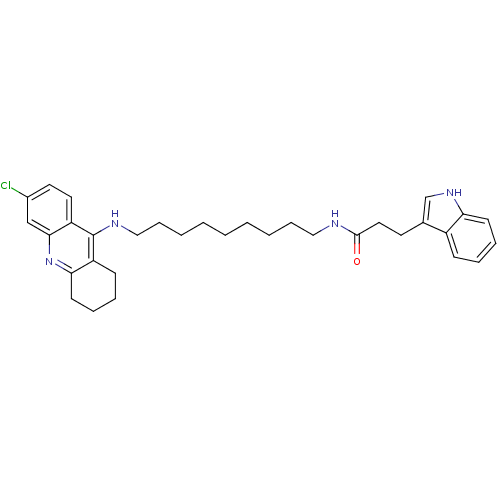 (Indole-Tacrine Heterodimer 8 | N-[9-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9038 (Indole-Tacrine Heterodimer 21 | N-[6-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9024 (Indole-Tacrine Heterodimer 7 | N-[8-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9070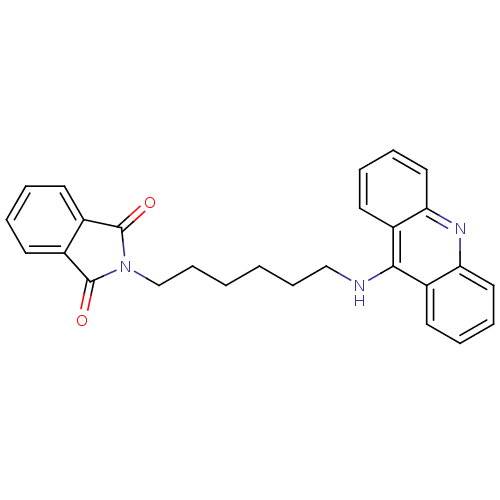 (2-[6-(Acridin-9-ylamino)-hexyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9031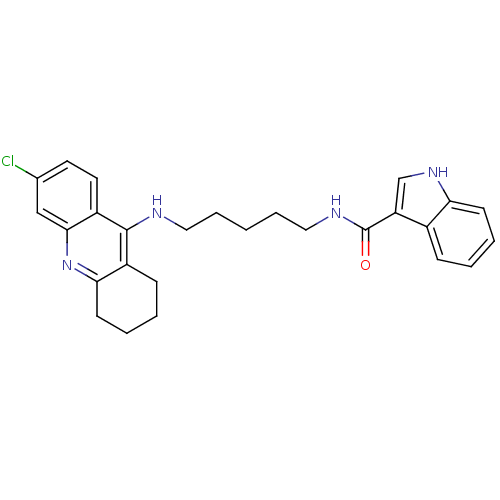 (1H-Indole-3-carboxylic Acid [5-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9025 (Indole-Tacrine Heterodimer 8 | N-[9-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9068 (2-[7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9065 (Donepezil-tacrine hybrid 10 | N-[6-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9039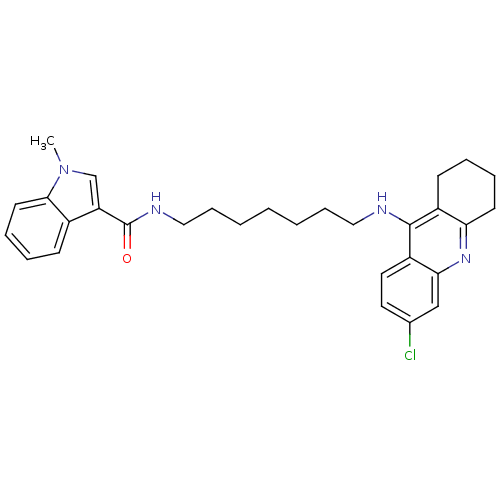 (1H-Methylindole-3-carboxylic Acid [7-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9035 (Indole-Tacrine Heterodimer 18 | N-[7-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9029 (Indole-Tacrine Heterodimer 12 | N-[6-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9021 (Indole-Tacrine Heterodimer 4 | N-[5-(6-Chloro-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM119688 (US8686042, 1-Benzyl-3-naphthalen-1-yl-urea) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.1 | n/a | n/a | n/a | n/a | 7.3 | 30 |
Neuropharma, S.A. US Patent | Assay Description The GSK-3β activity was determined by incubation of a mixture of recombinant human GSK-3 enzyme, a phosphate source and GSK-3 substrate in the p... | US Patent US8686042 (2014) BindingDB Entry DOI: 10.7270/Q2H993V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9030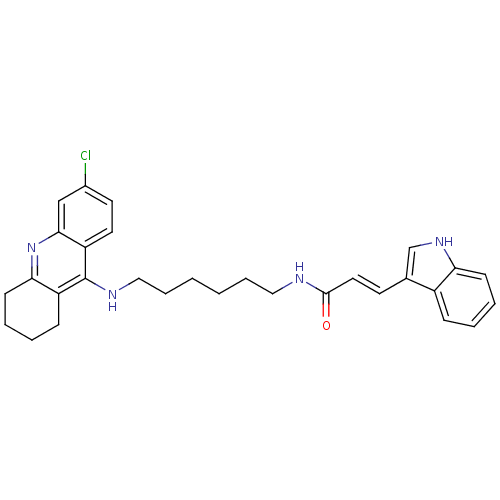 ((2E)-N-{6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9033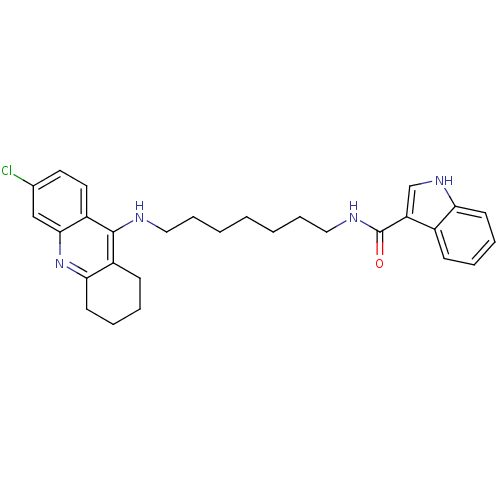 (1H-Indole-3-carboxylic Acid [7-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9066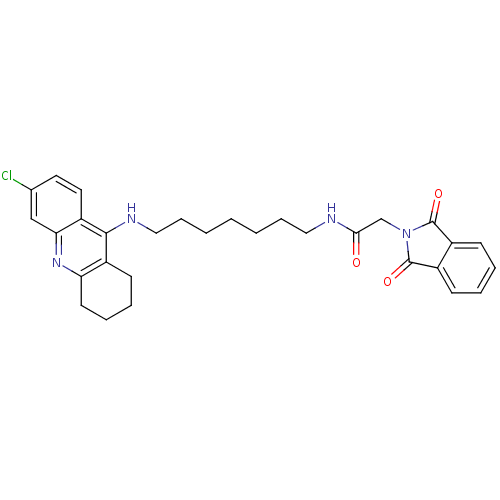 (Donepezil-tacrine hybrid 11 | N-[7-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9028 (Indole-Tacrine Heterodimer 11 | N-(3-{[3-(6-Chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9026 (Indole-Tacrine Heterodimer 9 | N-[10-(6-Chloro-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21.9 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9034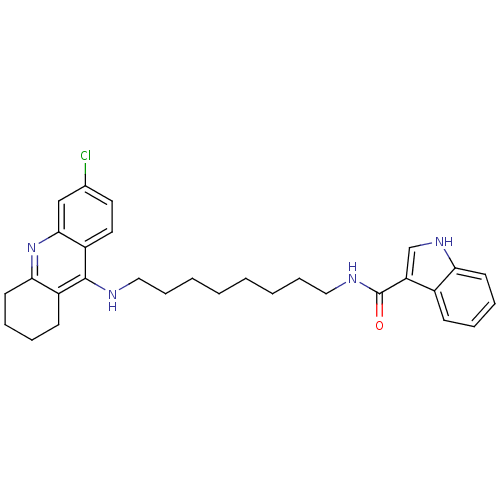 (1H-Indole-3-carboxylic Acid [8-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9063 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9069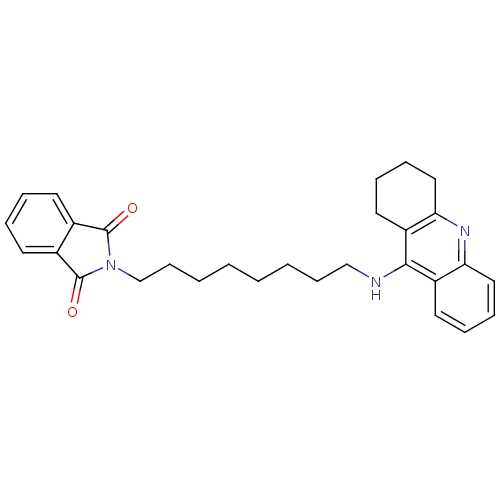 (2-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9032 (1H-Indole-3-carboxylic Acid [6-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9033 (1H-Indole-3-carboxylic Acid [7-(6-Chloro-1,2,3,4-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 5 min with ... | J Med Chem 48: 7223-33 (2005) Article DOI: 10.1021/jm0503289 BindingDB Entry DOI: 10.7270/Q2QN64Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM119689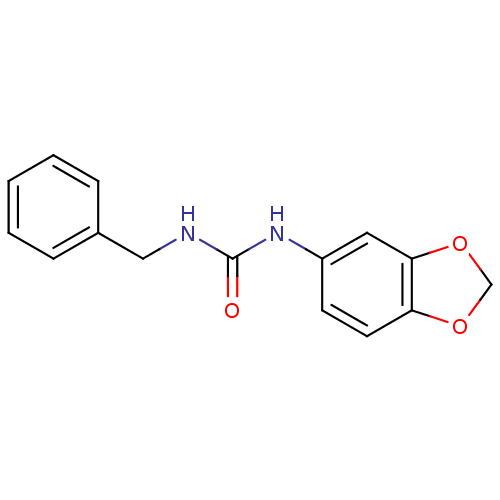 (US8686042, 1-Benzo[1,3]dioxol-5-yl-3-benzyl-urea) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38.4 | n/a | n/a | n/a | n/a | 7.3 | 30 |
Neuropharma, S.A. US Patent | Assay Description The GSK-3β activity was determined by incubation of a mixture of recombinant human GSK-3 enzyme, a phosphate source and GSK-3 substrate in the p... | US Patent US8686042 (2014) BindingDB Entry DOI: 10.7270/Q2H993V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |