

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitinase B (Serratia marcescens) | BDBM10854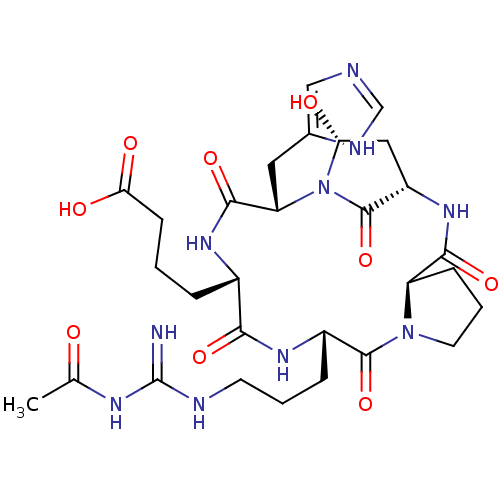 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Binding affinity to Serratia marcescens chitinase B | Bioorg Med Chem 16: 3565-79 (2008) Article DOI: 10.1016/j.bmc.2008.02.017 BindingDB Entry DOI: 10.7270/Q2NG4QCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50611491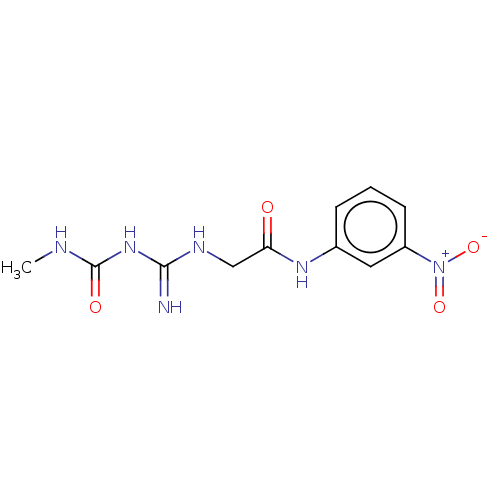 (CHEMBL5290116) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50214361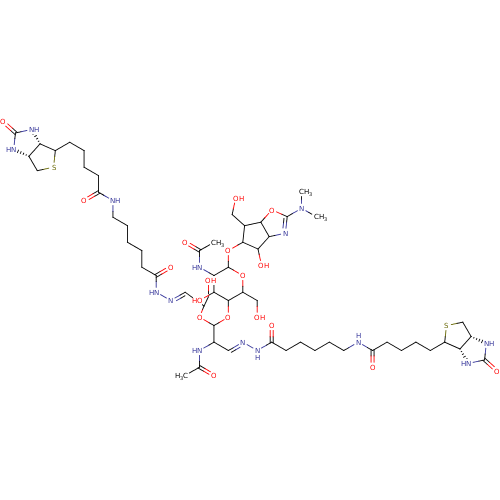 (CHEMBL415389) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.53 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trichoderma Chitinase | Bioorg Med Chem Lett 8: 2987-90 (1998) BindingDB Entry DOI: 10.7270/Q2TM7D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50611492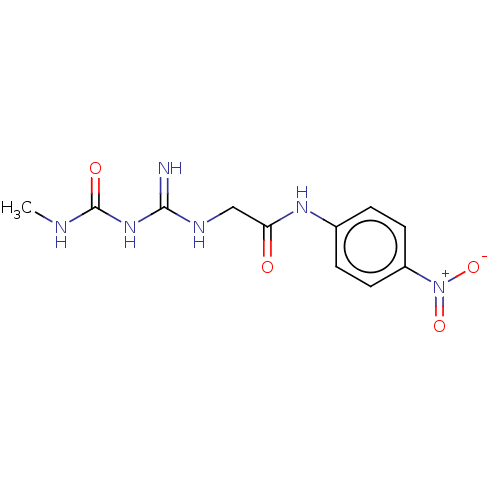 (CHEMBL5289869) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | UniChem | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM10854 (4-[(1S,4R,10S,13S,16S,18R)-10-{3-[(acetamidomethan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 5.2 | 37 |
University of Dundee | Assay Description The IC50s of inhibitor against the human chitinase were determined using the fluorogenic substrate 4MU-NAG3. The fluorescence of the liberated 4MU wa... | Chem Biol 12: 65-76 (2005) Article DOI: 10.1016/j.chembiol.2004.10.013 BindingDB Entry DOI: 10.7270/Q23F4MV6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554342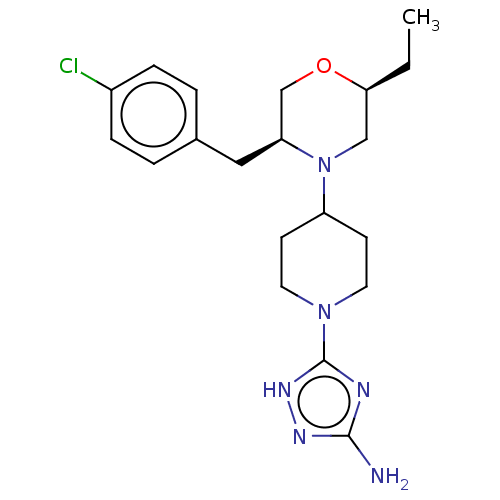 (CHEMBL4776610) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554344 (CHEMBL4756869) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50214358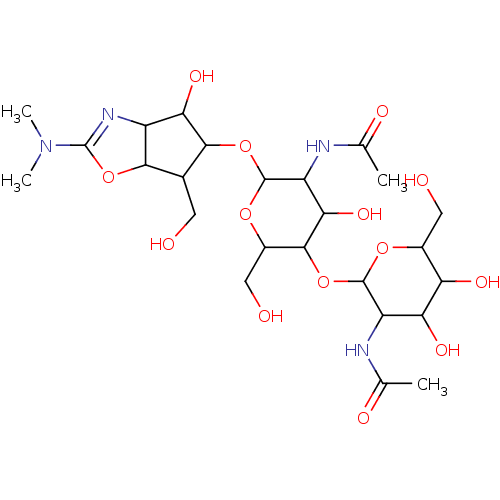 (CHEMBL318258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 16.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trichoderma Chitinase | Bioorg Med Chem Lett 8: 2987-90 (1998) BindingDB Entry DOI: 10.7270/Q2TM7D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554343 (CHEMBL4794014) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504025 (CHEMBL4464754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554347 (CHEMBL4755139) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50462584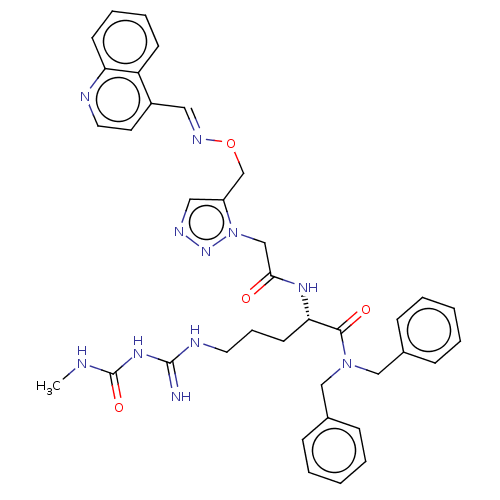 (CHEMBL4245260) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Serratia marcescens chitinase B | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554345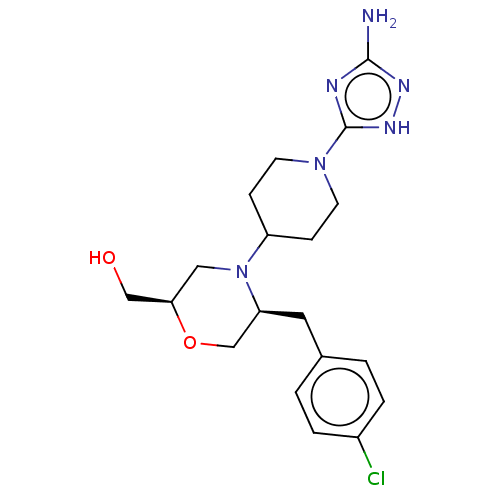 (CHEMBL4789376) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50462584 (CHEMBL4245260) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens 6x-His-tagged ChiB after 20 hrs by LCMS-SIR analysis | J Med Chem 61: 9395-9409 (2018) Article DOI: 10.1021/acs.jmedchem.8b00266 BindingDB Entry DOI: 10.7270/Q2765J0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554340 (CHEMBL4788866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554346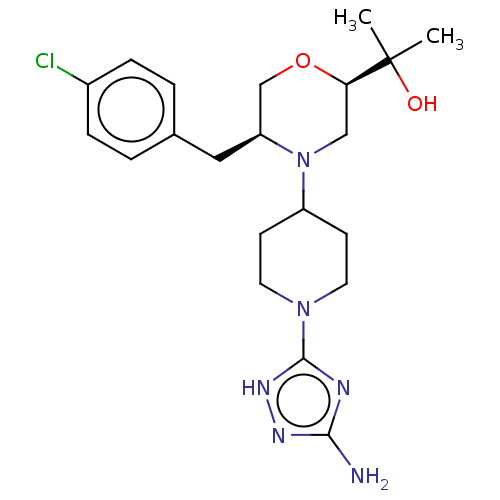 (CHEMBL4764430) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM10853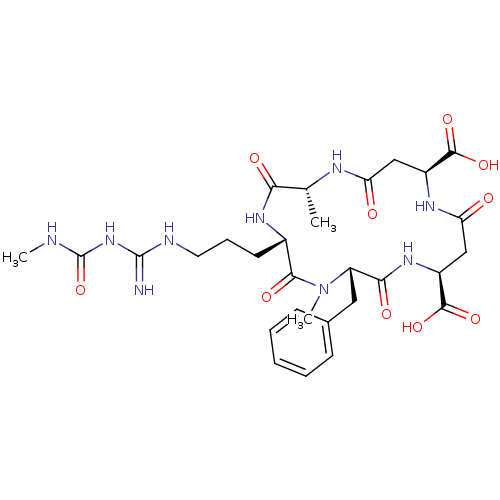 ((2R,5S,8S,11S,15S)-8-benzyl-2,7-dimethyl-5-[3-({[(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of Dundee | Assay Description Inhibition of argifin and other peptide derivatives against AfchiB1 and hCHT. | Chem Biol 15: 295-301 (2008) Article DOI: 10.1016/j.chembiol.2008.02.015 BindingDB Entry DOI: 10.7270/Q2JH3JN1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endochitinase B1 (Aspergillus fumigatus) | BDBM50559069 (CHEMBL4756641) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Aspergillus fumigatus chitinase B1 expressed in Escherichia coli using 4MU-GlcNAc2 as substrate by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00748 BindingDB Entry DOI: 10.7270/Q2348Q30 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504017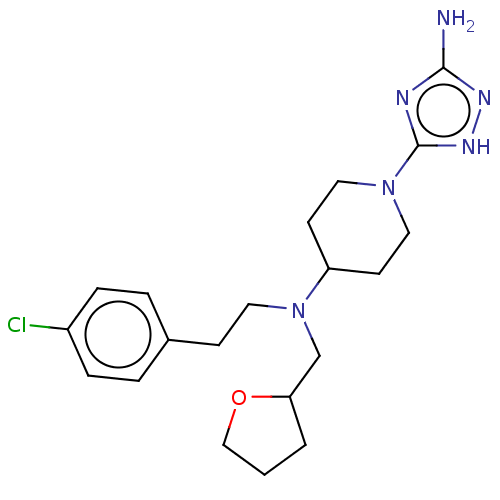 (CHEMBL4541831) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50554348 (CHEMBL4749391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089857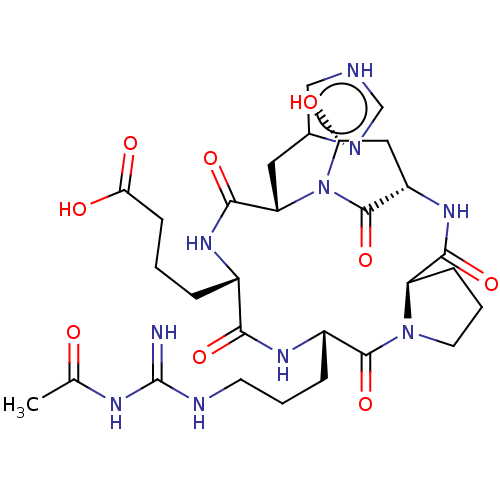 (Argadin) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243685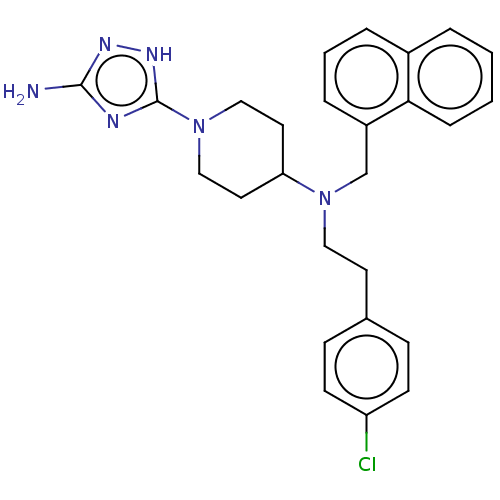 (CHEMBL4084573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50089847 (CHEMBL3577620) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504019 (CHEMBL4580568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504019 (CHEMBL4580568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50214357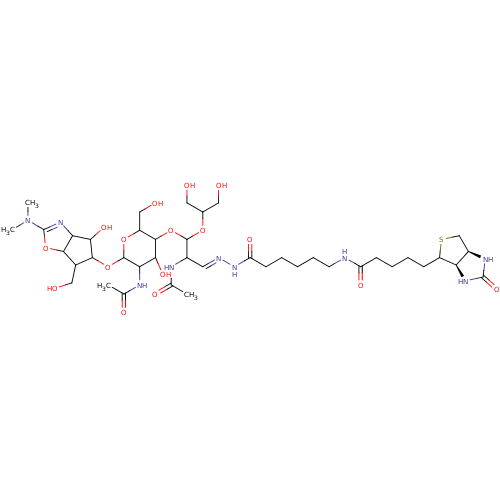 (CHEMBL319102) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trichoderma Chitinase | Bioorg Med Chem Lett 8: 2987-90 (1998) BindingDB Entry DOI: 10.7270/Q2TM7D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50214360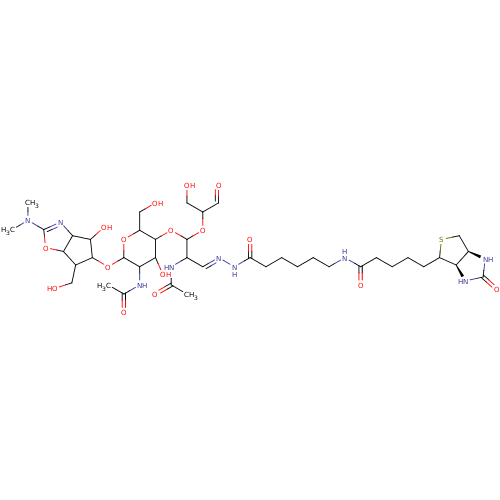 (CHEMBL327266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 41.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trichoderma Chitinase | Bioorg Med Chem Lett 8: 2987-90 (1998) BindingDB Entry DOI: 10.7270/Q2TM7D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504037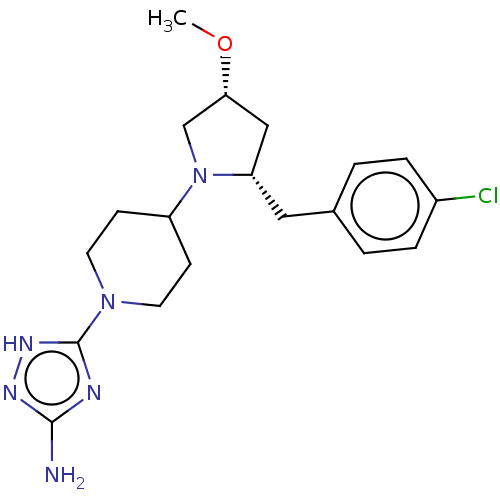 (CHEMBL4470253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504028 (CHEMBL4520755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504016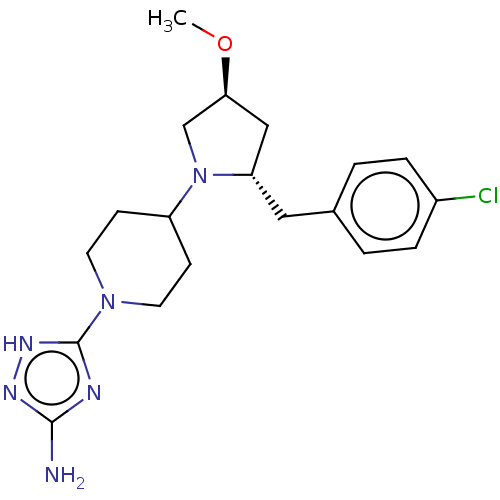 (CHEMBL4537828) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504024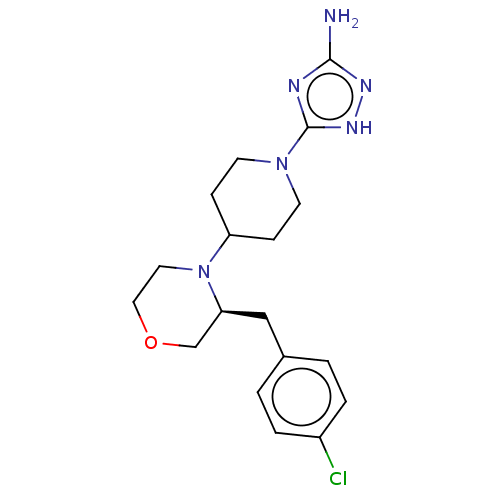 (CHEMBL4461925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human recombinant CHIT1 catalytic domain (1 to 386 residues) expressed in HEK293F cells assessed as r... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504024 (CHEMBL4461925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50541930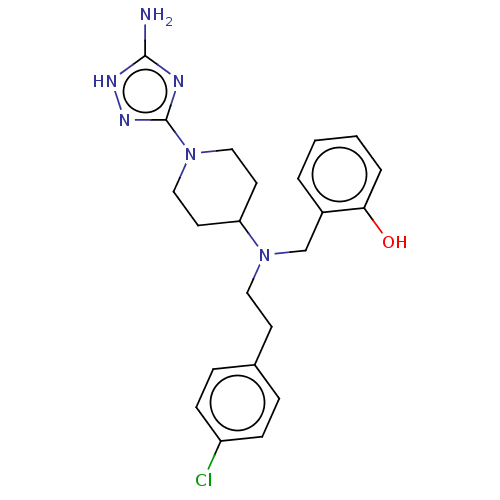 (CHEMBL4637417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-taged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using ... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504034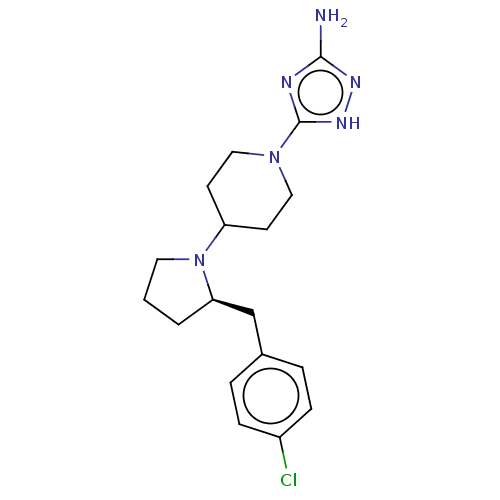 (CHEMBL4588384) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50541931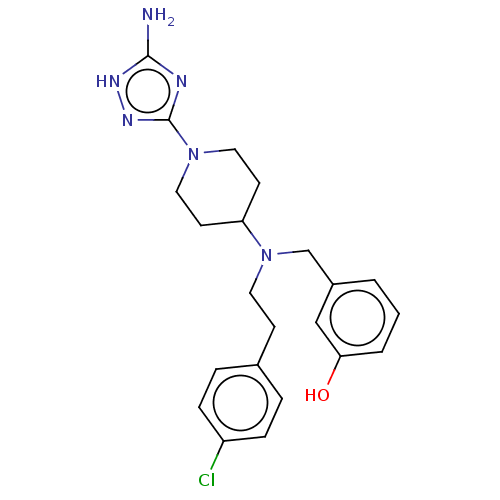 (CHEMBL4634943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-taged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using ... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50214359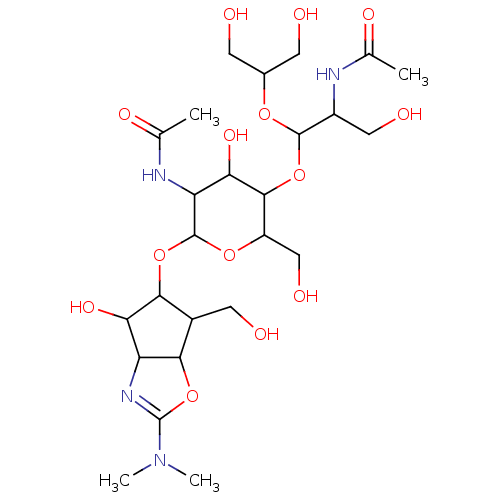 (CHEMBL103401) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Trichoderma Chitinase | Bioorg Med Chem Lett 8: 2987-90 (1998) BindingDB Entry DOI: 10.7270/Q2TM7D9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504039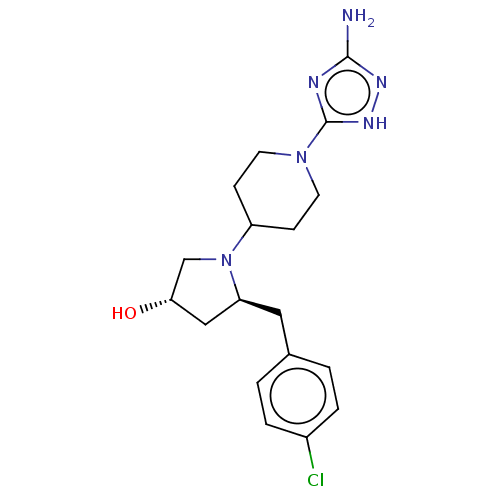 (CHEMBL4442663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504031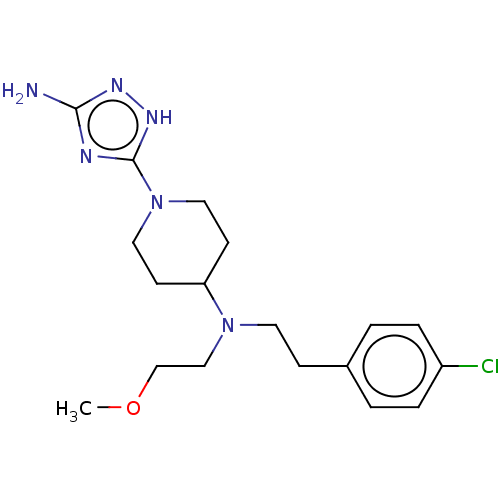 (CHEMBL4521547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50504014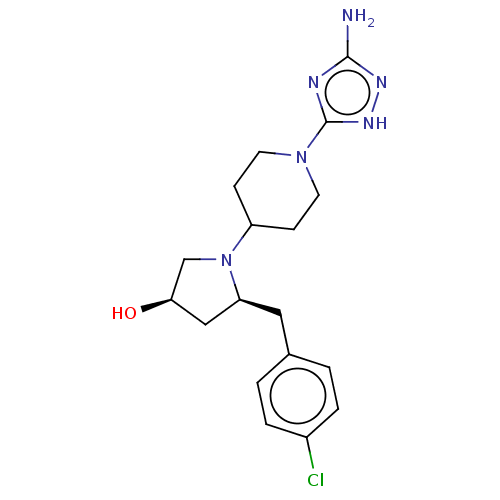 (CHEMBL4547227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... | J Med Chem 62: 7126-7145 (2019) Article DOI: 10.1021/acs.jmedchem.9b00681 BindingDB Entry DOI: 10.7270/Q27H1NTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50541933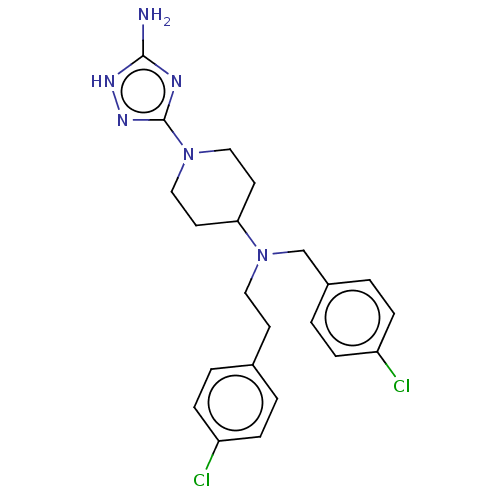 (CHEMBL4646309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics S.A. Curated by ChEMBL | Assay Description Inhibition of full length recombinant C-terminal His-taged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using ... | ACS Med Chem Lett 11: 1228-1235 (2020) Article DOI: 10.1021/acsmedchemlett.0c00092 BindingDB Entry DOI: 10.7270/Q2PV6PX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50331851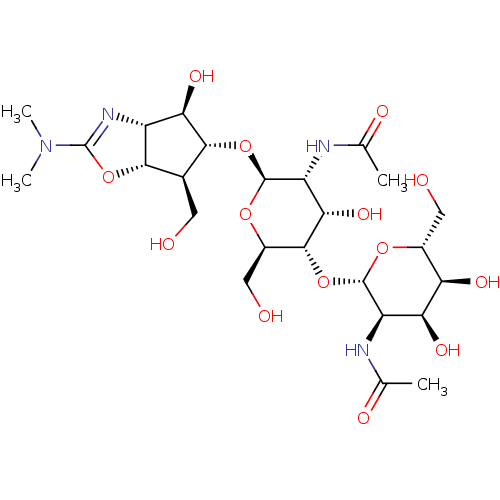 (Allosamidin | CHEMBL1230997) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Inhibition of Serratia marcescens chitinase ChiB assessed as reduction in chitinolytic activity using 4MU-(GlcNAc)2 substrate by fluorescence based a... | J Med Chem 58: 4984-97 (2015) Article DOI: 10.1021/acs.jmedchem.5b00175 BindingDB Entry DOI: 10.7270/Q2VT1TV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitinase B (Serratia marcescens) | BDBM50257241 (CHEMBL506684 | N-Ac-D-Ala-Arg{N-omega-(N-methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University Curated by ChEMBL | Assay Description Displacement of 4-methylumbelliferydiacetyl-chitobiose from Serratia marcescens ChiB | Bioorg Med Chem 17: 2751-8 (2009) Article DOI: 10.1016/j.bmc.2009.02.047 BindingDB Entry DOI: 10.7270/Q2PR7VVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601600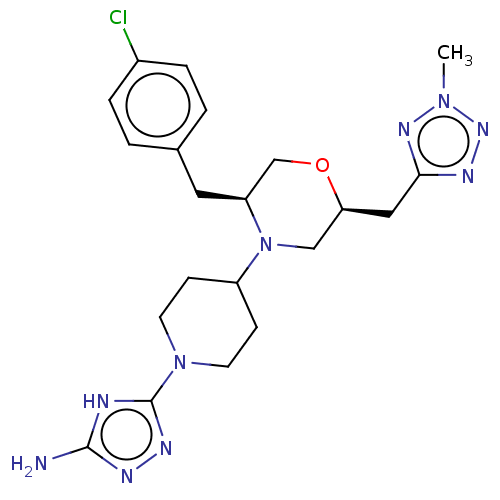 (US11638707, Example 5.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601601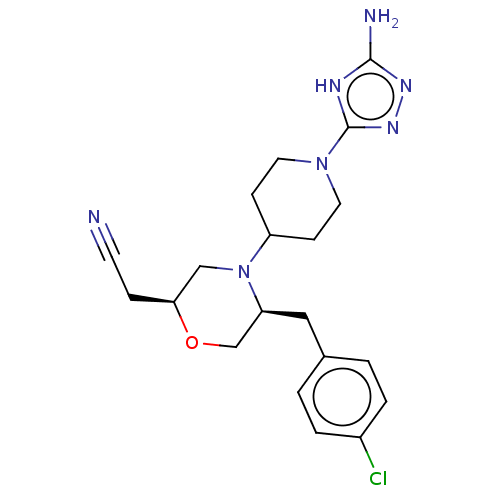 (US11638707, Example 6.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601602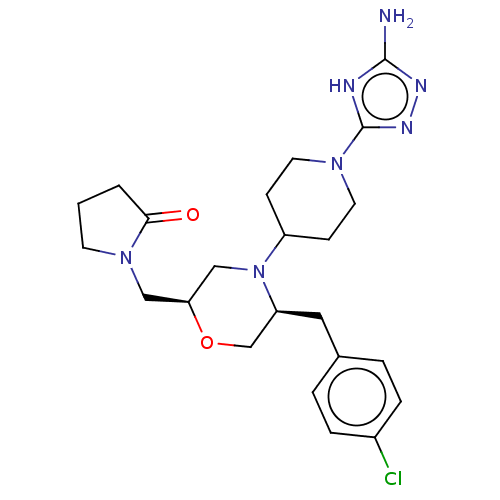 (US11638707, Example 7.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601603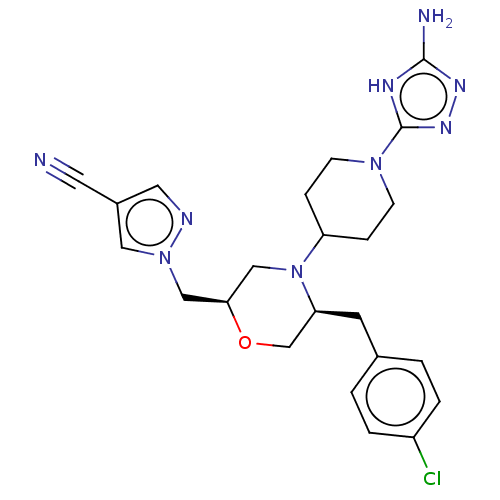 (US11638707, Example 8.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601604 (US11638707, Example 9.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601605 (US11638707, Example 10.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601606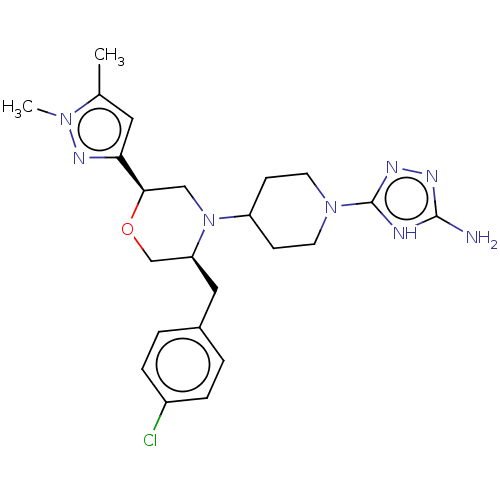 (US11638707, Example 11.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM601607 (US11638707, Example 12.) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2DV1PTC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 478 total ) | Next | Last >> |