

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-5 (Homo sapiens (Human)) | BDBM50160974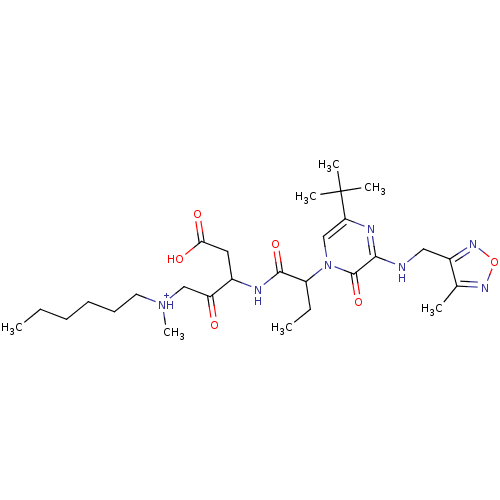 (CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against human caspase-5 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50160957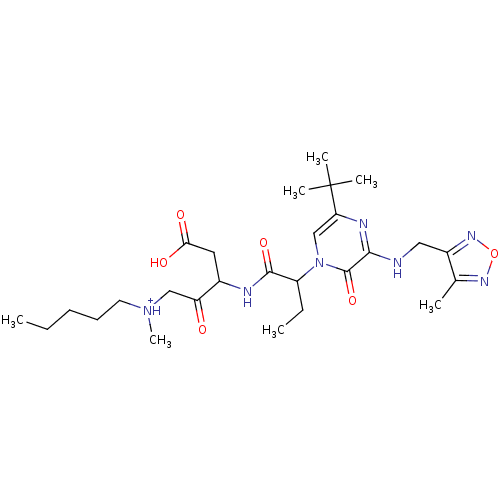 (CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against casp-5 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200547 (CHEMBL3949842) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200556 (CHEMBL3904435) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200544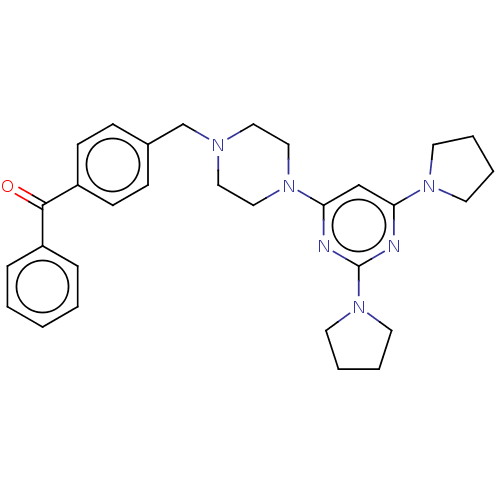 (CHEMBL3970645) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200545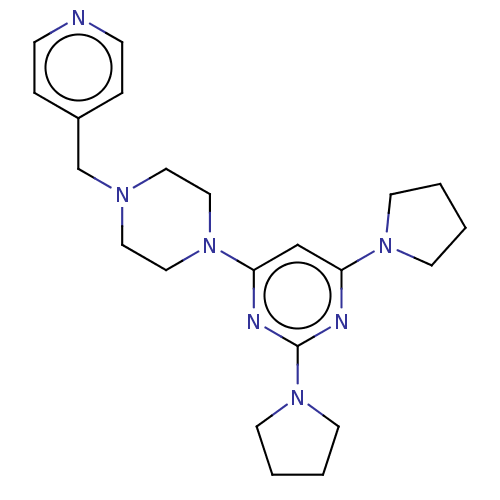 (CHEMBL3965572) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200543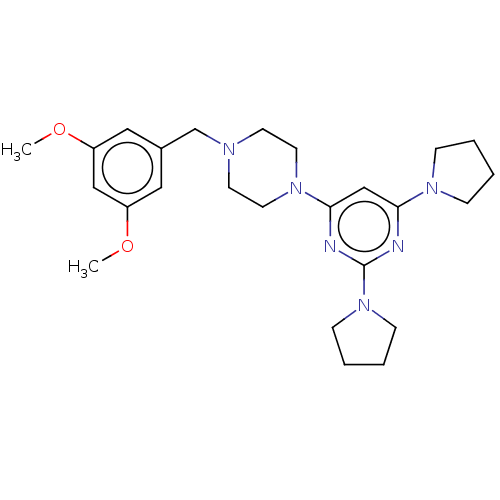 (CHEMBL3941088) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200548 (CHEMBL3895496) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 45 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200549 (CHEMBL3932441) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200550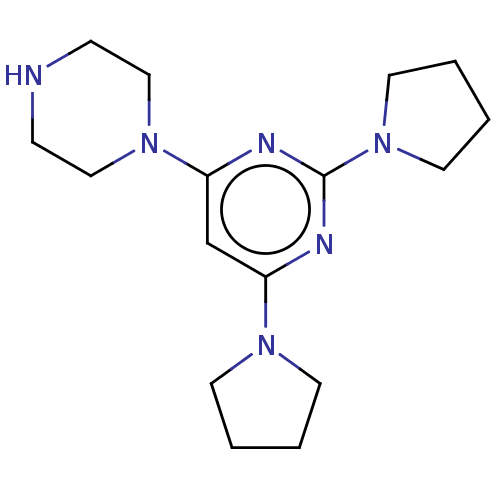 (CHEMBL3905486) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200552 (CHEMBL3898411) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200548 (CHEMBL3895496) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200551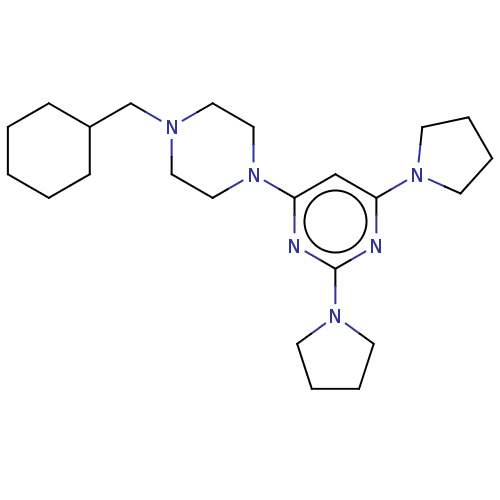 (CHEMBL3978901) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200553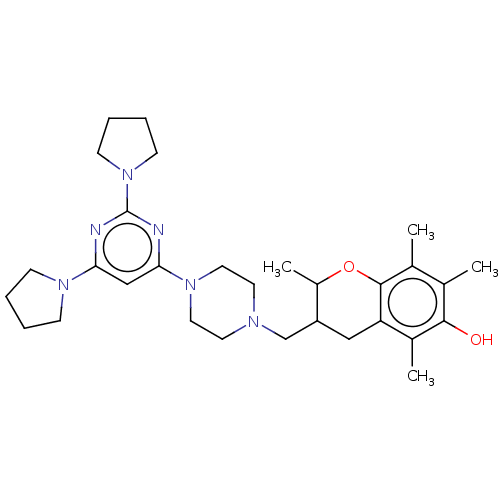 (CHEMBL3898379) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200555 (CHEMBL3984293) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM223313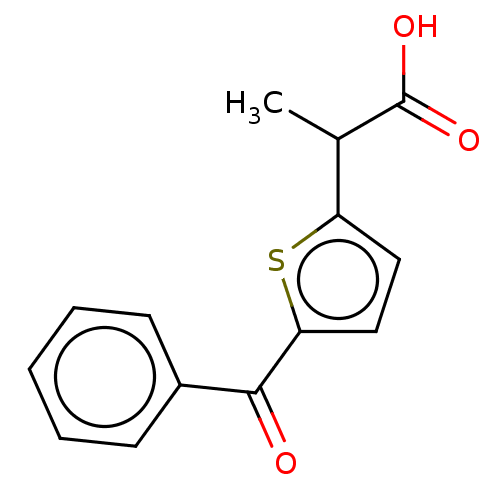 (Tiaprofenic acid) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Colorado | Assay Description Experiments were performed in a 384-well format (Greiner no. 781207) as per the conditions noted here. Caspase-1: 2.5 nM enzyme, 6.5 mM WEHD substrat... | Cell Chem Biol 24: 281-292 (2017) Article DOI: 10.1016/j.chembiol.2017.02.003 BindingDB Entry DOI: 10.7270/Q2C53JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200546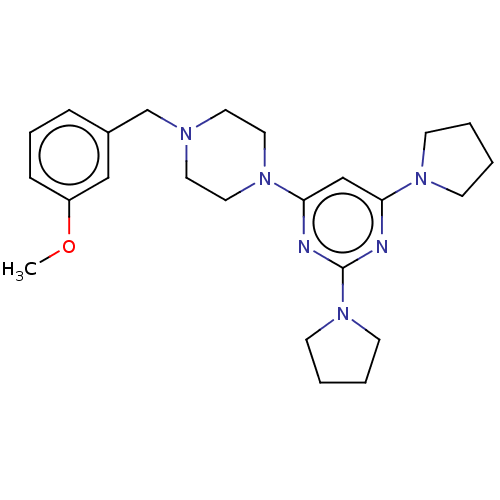 (CHEMBL3939229) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50426564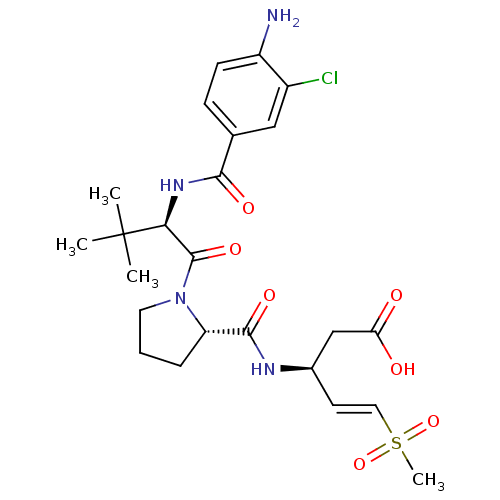 (CHEMBL2323966) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of caspase-5 (unknown origin) | ACS Med Chem Lett 4: 163-4 (2013) Article DOI: 10.1021/ml400021b BindingDB Entry DOI: 10.7270/Q2736S86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200554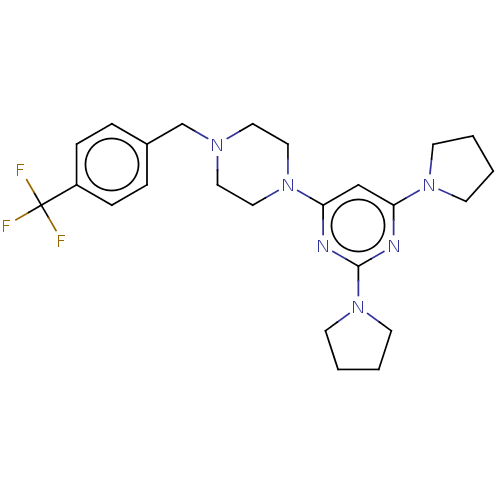 (CHEMBL3968765) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 586 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50233673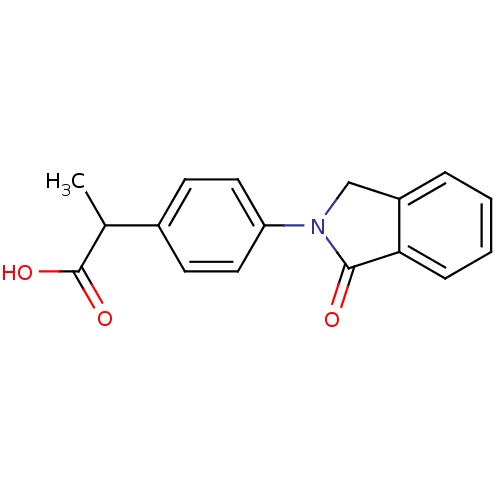 ((+)-2-(4-(1-oxoisoindolin-2-yl)phenyl)propanoic ac...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Colorado | Assay Description Experiments were performed in a 384-well format (Greiner no. 781207) as per the conditions noted here. Caspase-1: 2.5 nM enzyme, 6.5 mM WEHD substrat... | Cell Chem Biol 24: 281-292 (2017) Article DOI: 10.1016/j.chembiol.2017.02.003 BindingDB Entry DOI: 10.7270/Q2C53JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50426565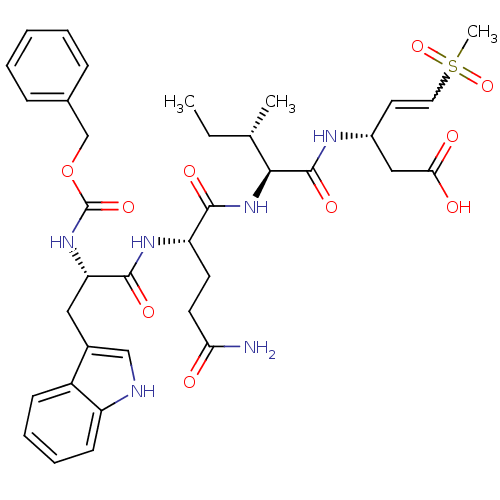 (CHEMBL2324340) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of caspase-5 (unknown origin) | ACS Med Chem Lett 4: 163-4 (2013) Article DOI: 10.1021/ml400021b BindingDB Entry DOI: 10.7270/Q2736S86 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50240374 (3-(4-biphenylylcarbonyl)propionic acid | 3-(4-phen...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Colorado | Assay Description Experiments were performed in a 384-well format (Greiner no. 781207) as per the conditions noted here. Caspase-1: 2.5 nM enzyme, 6.5 mM WEHD substrat... | Cell Chem Biol 24: 281-292 (2017) Article DOI: 10.1016/j.chembiol.2017.02.003 BindingDB Entry DOI: 10.7270/Q2C53JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM166683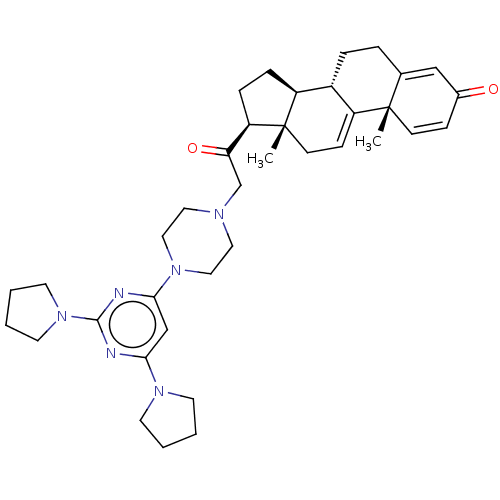 (U74389G (B9)) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 30 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM85511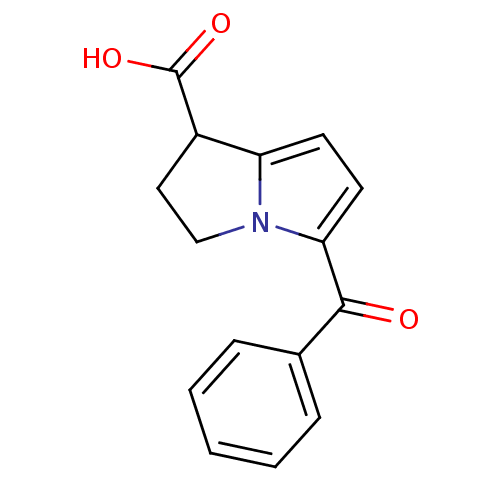 (CAS_74103-07-4 | KETOROLAC | Ketorolac tris salt |...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Colorado | Assay Description Experiments were performed in a 384-well format (Greiner no. 781207) as per the conditions noted here. Caspase-1: 2.5 nM enzyme, 6.5 mM WEHD substrat... | Cell Chem Biol 24: 281-292 (2017) Article DOI: 10.1016/j.chembiol.2017.02.003 BindingDB Entry DOI: 10.7270/Q2C53JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50200548 (CHEMBL3895496) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DePaul University Curated by ChEMBL | Assay Description Inhibition of human recombinant DTT-activated caspase-5 expressed in Escherichia coli using Ac-WEHD-AMC as substrate preincubated for 15 mins followe... | Bioorg Med Chem Lett 26: 5476-5480 (2016) Article DOI: 10.1016/j.bmcl.2016.10.025 BindingDB Entry DOI: 10.7270/Q2NZ89M0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM223312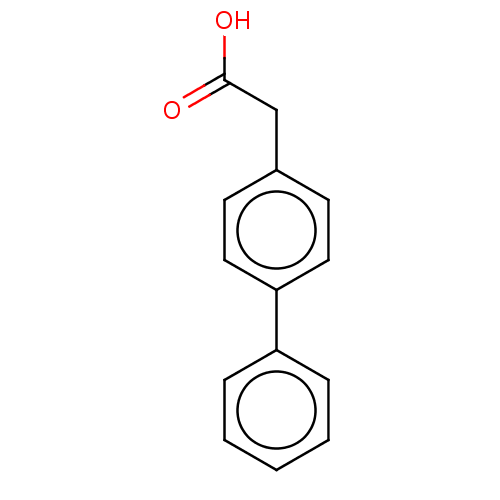 (Felbinac) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Colorado | Assay Description Experiments were performed in a 384-well format (Greiner no. 781207) as per the conditions noted here. Caspase-1: 2.5 nM enzyme, 6.5 mM WEHD substrat... | Cell Chem Biol 24: 281-292 (2017) Article DOI: 10.1016/j.chembiol.2017.02.003 BindingDB Entry DOI: 10.7270/Q2C53JQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM50453034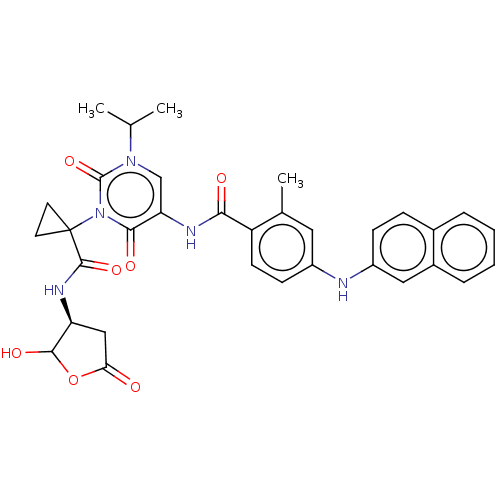 (CHEMBL4211859) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nestl� Skin Health R&D Curated by ChEMBL | Assay Description Inhibition of human recombinant caspase-5 using AC-WEHD-AMC as substrate at Km after 20 mins by fluorescence assay | J Med Chem 61: 4030-4051 (2018) Article DOI: 10.1021/acs.jmedchem.8b00067 BindingDB Entry DOI: 10.7270/Q2FR006X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160813 (US10167313, Compound 82 | US9045524, 82) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160813 (US10167313, Compound 82 | US9045524, 82) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160784 (US10167313, Compound 51 | US9045524, 51) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160784 (US10167313, Compound 51 | US9045524, 51) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160783 (US10167313, Compound 50 | US9045524, 50) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160786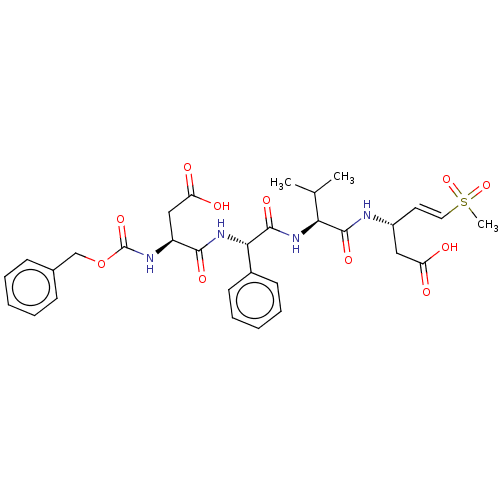 (US10167313, Compound 53 | US9045524, 53) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160770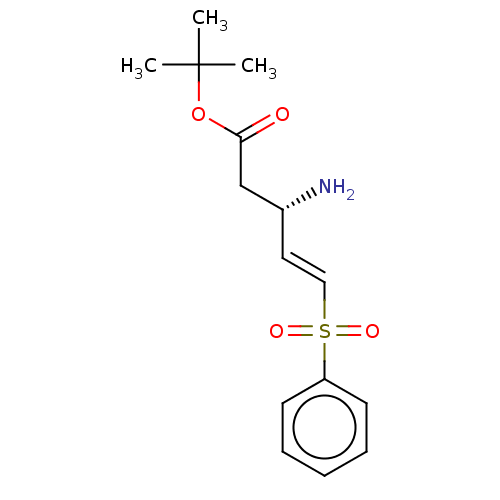 (US10167313, Compound 37 | US9045524, 37) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160750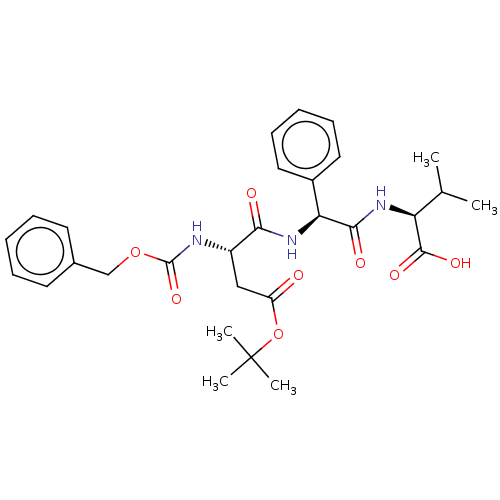 (US10167313, Compound 16 | US9045524, 16) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160801 (US10167313, Compound 68 | US9045524, 68) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160769 (US10167313, Compound 36 | US9045524, 36) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160841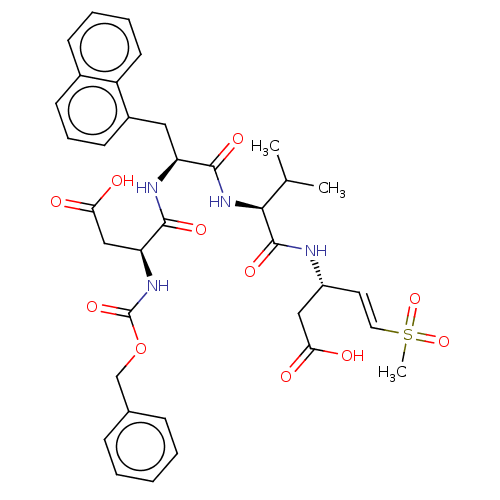 (US9045524, 111) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160849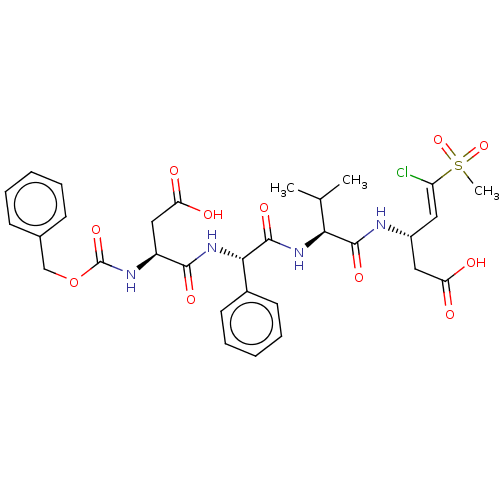 (US9045524, 123) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160781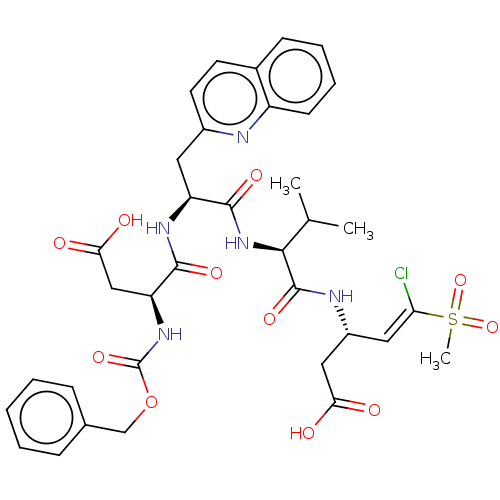 (US10167313, Compound 48 | US9045524, 48) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NOVAGENESIS FOUNDATION US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160794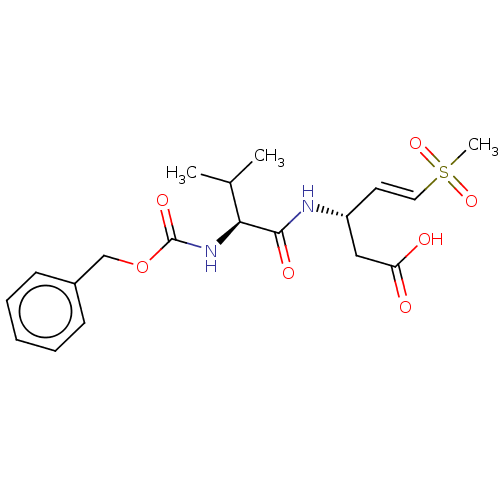 (US10167313, Compound 61 | US9045524, 61) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160792 (US10167313, Compound 59 | US9045524, 59) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160819 (US10167313, Compound 88 | US9045524, 88) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160790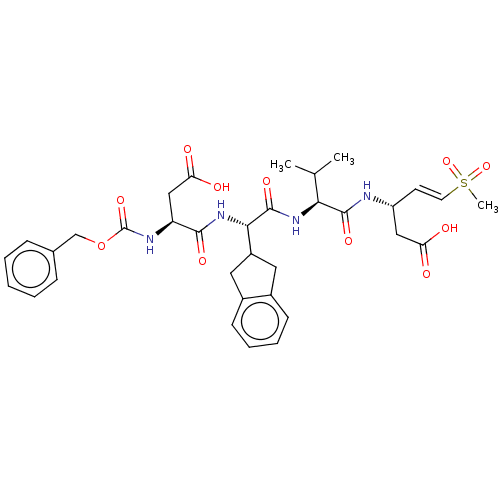 (US10167313, Compound 57 | US9045524, 57) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160808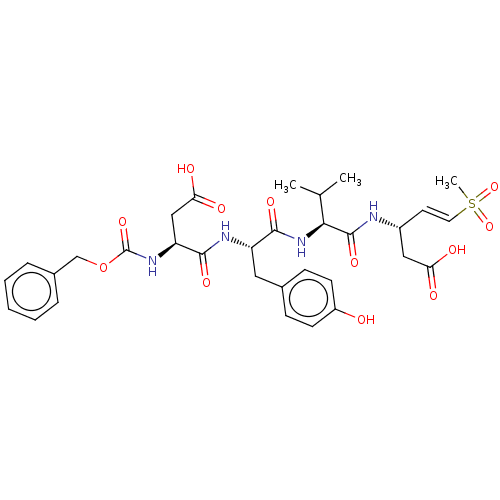 (US10167313, Compound 76 | US9045524, 76) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160816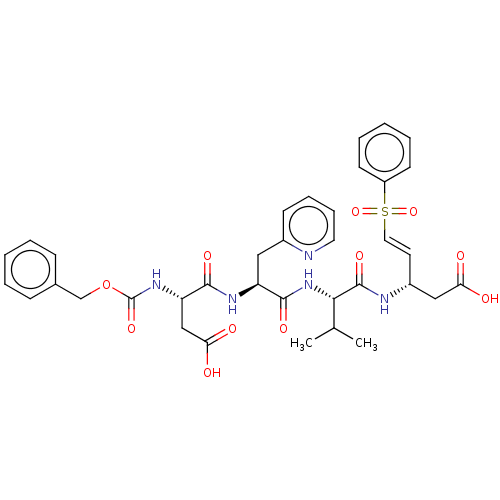 (US10167313, Compound 85 | US9045524, 85) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160796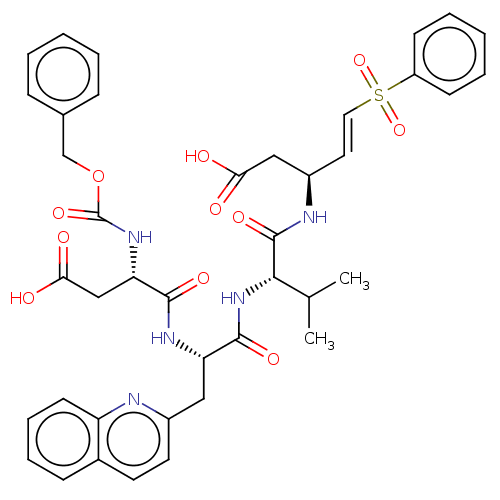 (US10167313, Compound 63 | US9045524, 63) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160798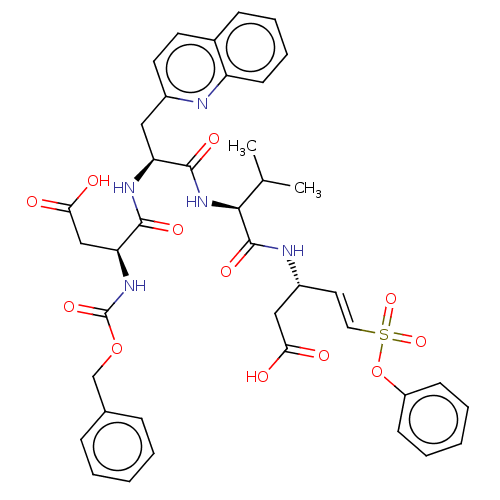 (US10167313, Compound 65 | US9045524, 65) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160827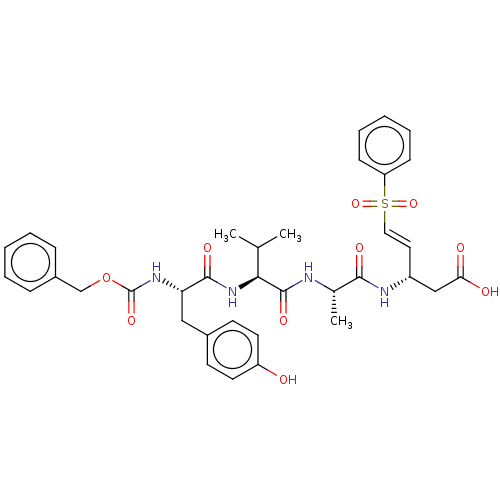 (US10167313, Compound 96 | US9045524, 96) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-5 (Homo sapiens (Human)) | BDBM160781 (US10167313, Compound 48 | US9045524, 48) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Technologies Limited US Patent | Assay Description Selectivity of compound 55, compound 63, compound 48, compound 57, compound 88 toward caspase-1 (pro-inflammatory group), caspase-5 (group I), caspas... | US Patent US10167313 (2019) BindingDB Entry DOI: 10.7270/Q2Q81G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |