 Found 10533 hits of ic50 data for polymerid = 1967
Found 10533 hits of ic50 data for polymerid = 1967 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50574359
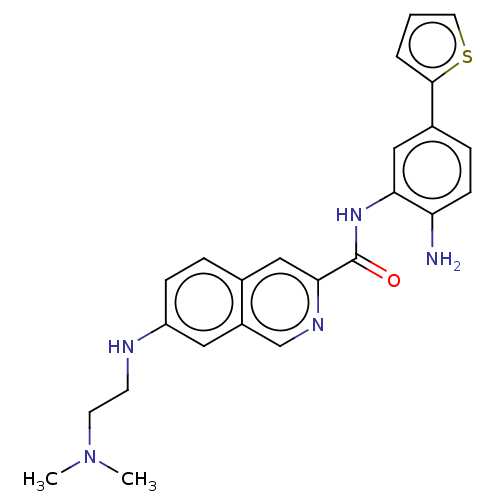
(CHEMBL4856052)Show SMILES CN(C)CCNc1ccc2cc(ncc2c1)C(=O)Nc1cc(ccc1N)-c1cccs1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 10 mins followed by tripeptide substrate addition and measured after 60 mins by fluorescence mi... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00488
BindingDB Entry DOI: 10.7270/Q2VH5SM1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50574358

(CHEMBL4860757)Show SMILES CN(C)CCN(C)c1ccc2cc(cnc2c1)C(=O)Nc1cc(ccc1N)-c1cccs1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) preincubated for 10 mins followed by tripeptide substrate addition and measured after 60 mins by fluorescence mi... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00488
BindingDB Entry DOI: 10.7270/Q2VH5SM1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM117170
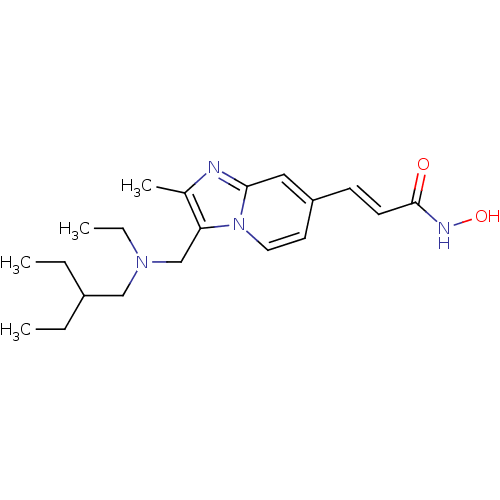
(US8648092, 101)Show SMILES CCC(CC)CN(CC)Cc1c(C)nc2cc(\C=C\C(=O)NO)ccn12 Show InChI InChI=1S/C20H30N4O2/c1-5-16(6-2)13-23(7-3)14-18-15(4)21-19-12-17(10-11-24(18)19)8-9-20(25)22-26/h8-12,16,26H,5-7,13-14H2,1-4H3,(H,22,25)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM117141
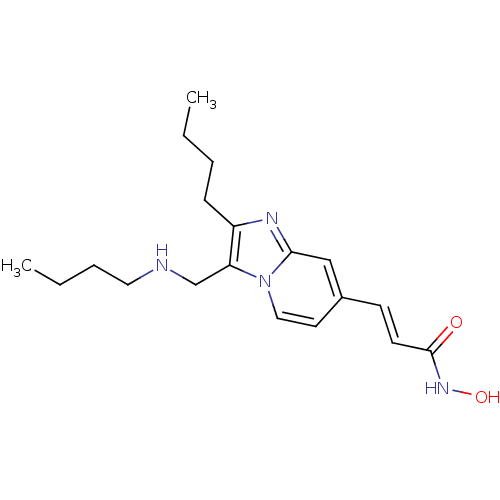
(US8648092, 72)Show InChI InChI=1S/C19H28N4O2/c1-3-5-7-16-17(14-20-11-6-4-2)23-12-10-15(13-18(23)21-16)8-9-19(24)22-25/h8-10,12-13,20,25H,3-7,11,14H2,1-2H3,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50573101
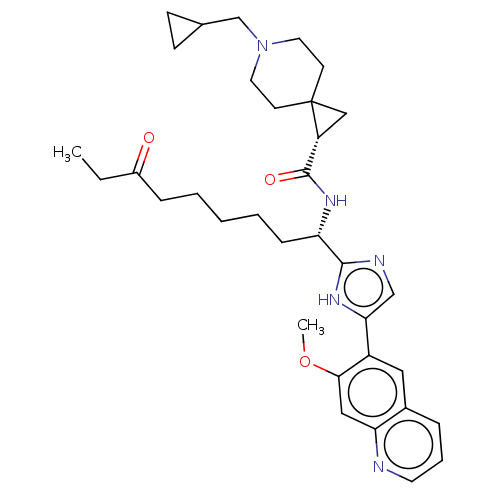
(CHEMBL4861467)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2cccnc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells incubated for 30 mins by Fluor De Lys assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00336
BindingDB Entry DOI: 10.7270/Q2DV1PPK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM117146

(US8648092, 77)Show SMILES CCCCN(CC)Cc1c(CCCC)nc2cc(\C=C\C(=O)NO)ccn12 Show InChI InChI=1S/C21H32N4O2/c1-4-7-9-18-19(16-24(6-3)13-8-5-2)25-14-12-17(15-20(25)22-18)10-11-21(26)23-27/h10-12,14-15,27H,4-9,13,16H2,1-3H3,(H,23,26)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged HDAC1 expressed in baculovirus infected Sf9 insect cells Fluor-de-lys as substrate measured after 2 hrs by... |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544211

(CHEMBL4641682)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C24H28FN5O3/c1-30-14-16(15-30)24(32)28-20(9-3-2-4-10-22(31)19-11-12-33-29-19)23-26-13-21(27-23)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,26,27)(H,28,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543650
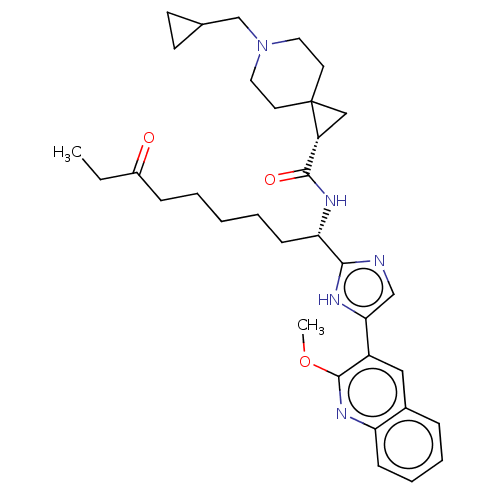
(CHEMBL4649205)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C34H45N5O3/c1-3-25(40)10-5-4-6-12-29(37-32(41)27-20-34(27)15-17-39(18-16-34)22-23-13-14-23)31-35-21-30(36-31)26-19-24-9-7-8-11-28(24)38-33(26)42-2/h7-9,11,19,21,23,27,29H,3-6,10,12-18,20,22H2,1-2H3,(H,35,36)(H,37,41)/t27-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544211

(CHEMBL4641682)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C24H28FN5O3/c1-30-14-16(15-30)24(32)28-20(9-3-2-4-10-22(31)19-11-12-33-29-19)23-26-13-21(27-23)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,26,27)(H,28,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length FLAG-tagged HDAC1 expressed in HEK293F cells using BML-KI104 as substrate in presence of SAHA as inhibito... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00462
BindingDB Entry DOI: 10.7270/Q2TH8RDR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC1 expressed in baculovirus infected sf9 cells using p53 residues 379-382 (RHKKAc) as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543649
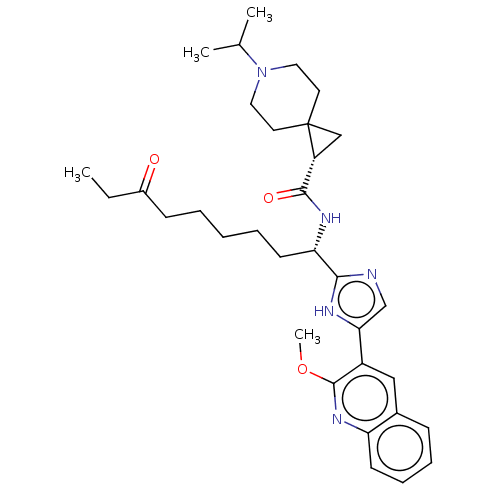
(CHEMBL4644038)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC1)C(C)C)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C33H45N5O3/c1-5-24(39)12-7-6-8-14-28(36-31(40)26-20-33(26)15-17-38(18-16-33)22(2)3)30-34-21-29(35-30)25-19-23-11-9-10-13-27(23)37-32(25)41-4/h9-11,13,19,21-22,26,28H,5-8,12,14-18,20H2,1-4H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568211
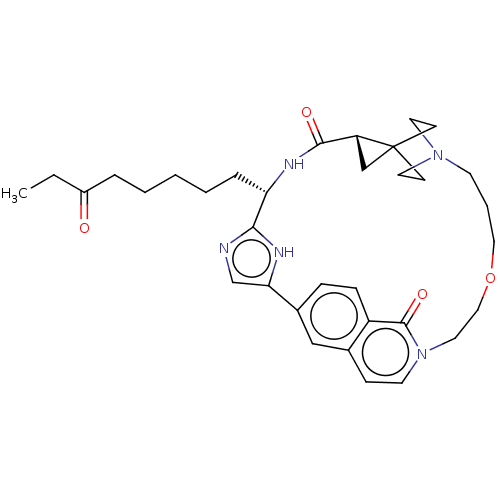
(CHEMBL4876610)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCOCCn2ccc3cc(ccc3c2=O)-c2cnc1[nH]2 |r,wU:13.12,wD:9.8,(53.94,-55.95,;52.55,-55.3,;51.3,-56.19,;51.44,-57.72,;49.9,-55.56,;48.65,-56.45,;47.25,-55.81,;45.99,-56.69,;44.61,-56.06,;43.35,-56.95,;43.49,-58.47,;44.88,-59.12,;46.14,-58.23,;45.04,-60.64,;45.93,-61.89,;44.4,-62.03,;43.02,-61.24,;41.66,-62.03,;41.66,-63.61,;43.03,-64.41,;44.41,-63.62,;40.26,-64.41,;38.55,-63.17,;36.55,-63.64,;33.33,-59.89,;30.34,-58.69,;30.85,-56.4,;32.42,-56.08,;32.92,-54.6,;34.44,-54.29,;35.47,-55.46,;36.96,-55.18,;37.98,-56.33,;37.49,-57.79,;35.99,-58.09,;34.97,-56.93,;33.45,-57.24,;32.94,-58.76,;39.48,-56.03,;40.12,-54.63,;41.65,-54.8,;41.95,-56.31,;40.62,-57.06,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568215
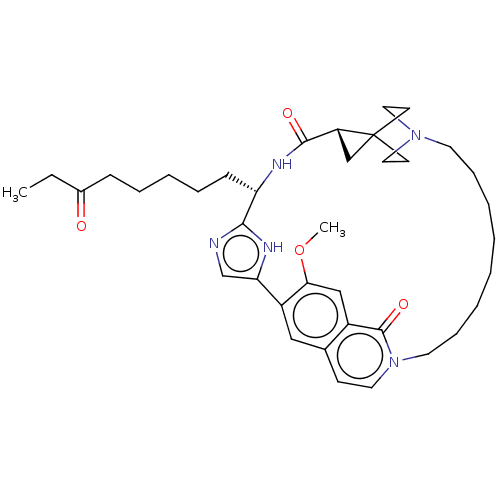
(CHEMBL4878197)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(-c4cnc1[nH]4)c(OC)cc3c2=O |r,wU:13.12,wD:9.8,(81.04,-4.01,;79.64,-3.37,;78.4,-4.27,;78.53,-5.8,;77,-3.63,;75.75,-4.52,;74.35,-3.88,;73.09,-4.76,;71.71,-4.12,;70.45,-5.02,;70.6,-6.54,;71.99,-7.19,;73.24,-6.29,;72.15,-8.71,;73.03,-9.95,;71.5,-10.1,;70.13,-9.31,;68.77,-10.1,;68.77,-11.67,;70.13,-12.47,;71.52,-11.68,;67.37,-12.47,;65.99,-11.67,;64.6,-12.47,;63.21,-11.22,;61.32,-11.48,;59.29,-7.36,;57.73,-6.26,;58.02,-4.74,;59.54,-4.15,;60.03,-2.67,;61.55,-2.37,;62.58,-3.53,;64.07,-3.25,;65.09,-4.4,;66.6,-4.1,;67.23,-2.7,;68.76,-2.88,;69.06,-4.38,;67.72,-5.13,;64.6,-5.85,;65.62,-7,;65.13,-8.45,;63.1,-6.16,;62.08,-5.01,;60.56,-5.31,;60.05,-6.83,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50531385
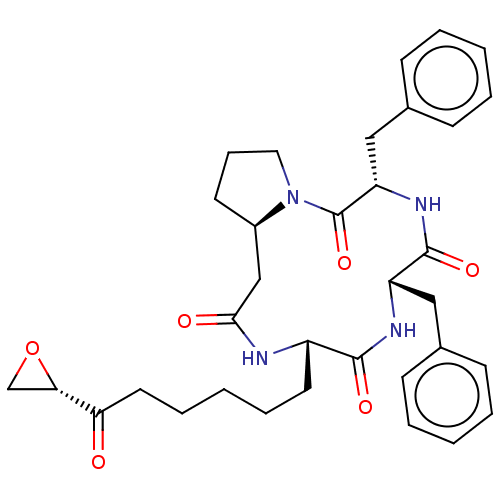
(CHEMBL4568666)Show SMILES [H][C@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCC(=O)[C@@H]1CO1)NC(=O)C2 |r| Show InChI InChI=1S/C34H42N4O6/c39-29(30-22-44-30)17-9-3-8-16-26-32(41)36-27(19-23-11-4-1-5-12-23)33(42)37-28(20-24-13-6-2-7-14-24)34(43)38-18-10-15-25(38)21-31(40)35-26/h1-2,4-7,11-14,25-28,30H,3,8-10,15-22H2,(H,35,40)(H,36,41)(H,37,42)/t25-,26+,27+,28+,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 expressed in NIH/3T3 cells using [3H]acetyl histone as substrate measured after 15 mins by liquid scintillation... |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113332
BindingDB Entry DOI: 10.7270/Q2F76HH8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human HDAC1 expressed in baculovirus infected Sf9 cells using RHKK-Ac as substrate by fluorescence analysis |
J Med Chem 59: 8967-9004 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00908
BindingDB Entry DOI: 10.7270/Q2KP8448 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327

(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full length C-terminal FLAG His-tagged human HDAC1 expressed in baculovirus infected Sf21 insect cells using RHK-K(Ac)-AMC ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02255
BindingDB Entry DOI: 10.7270/Q2JS9V8V |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50123617
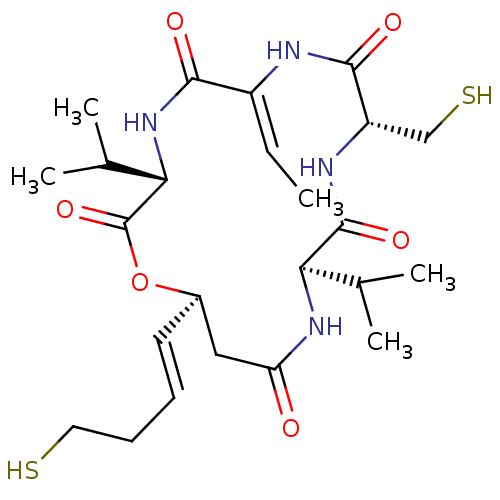
(CHEMBL3622726)Show SMILES C\C=C1/NC(=O)[C@H](CS)NC(=O)[C@H](NC(=O)C[C@H](OC(=O)[C@@H](NC1=O)C(C)C)\C=C\CCS)C(C)C |r| Show InChI InChI=1S/C24H38N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15(9-7-8-10-35)11-18(29)27-19(13(2)3)23(32)26-17(12-36)22(31)25-16/h6-7,9,13-15,17,19-20,35-36H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17+,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Institute of Biomedicine and Health
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC1 after 60 mins by fluorescence assay |
J Med Chem 58: 7672-80 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01044
BindingDB Entry DOI: 10.7270/Q2DF6T01 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50531393
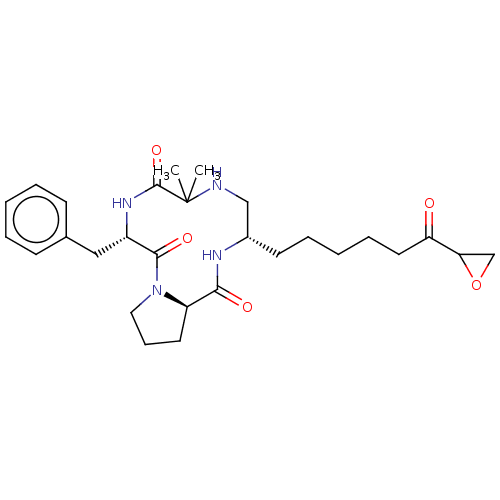
(CHEMBL4438063)Show SMILES [H][C@]12CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)C(C)(C)NC[C@H](CCCCCC(=O)C1CO1)NC2=O |r| Show InChI InChI=1S/C28H40N4O5/c1-28(2)27(36)31-21(16-19-10-5-3-6-11-19)26(35)32-15-9-13-22(32)25(34)30-20(17-29-28)12-7-4-8-14-23(33)24-18-37-24/h3,5-6,10-11,20-22,24,29H,4,7-9,12-18H2,1-2H3,(H,30,34)(H,31,36)/t20-,21-,22+,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem 18: 4103-10 (2010)
Article DOI: 10.1016/j.bmc.2010.03.080
BindingDB Entry DOI: 10.7270/Q2CC11N4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50470579
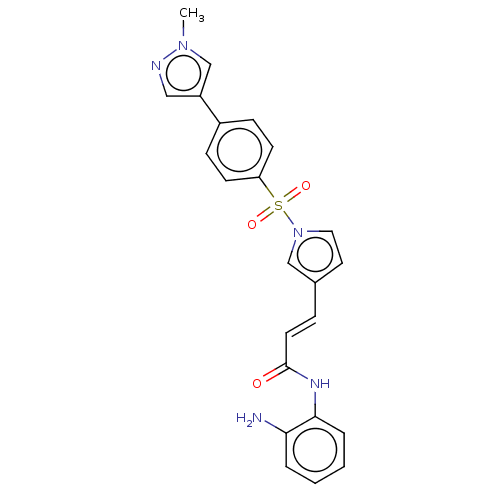
(4sc-202 | Domatinostat)Show SMILES Cn1cc(cn1)-c1ccc(cc1)S(=O)(=O)n1ccc(\C=C\C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C23H21N5O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24/h2-16H,24H2,1H3,(H,26,29)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544215
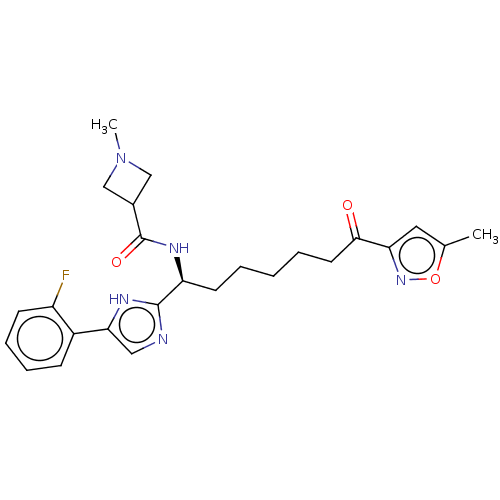
(CHEMBL4637689)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1cc(C)on1)c1ncc([nH]1)-c1ccccc1F |r| Show InChI InChI=1S/C25H30FN5O3/c1-16-12-21(30-34-16)23(32)11-5-3-4-10-20(29-25(33)17-14-31(2)15-17)24-27-13-22(28-24)18-8-6-7-9-19(18)26/h6-9,12-13,17,20H,3-5,10-11,14-15H2,1-2H3,(H,27,28)(H,29,33)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50573102

(CHEMBL4862152)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(CC2CC2)CC1)c1ncc(o1)-c1cc2ccc(C)nc2cc1OC |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells incubated for 30 mins by Fluor De Lys assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00336
BindingDB Entry DOI: 10.7270/Q2DV1PPK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50530002
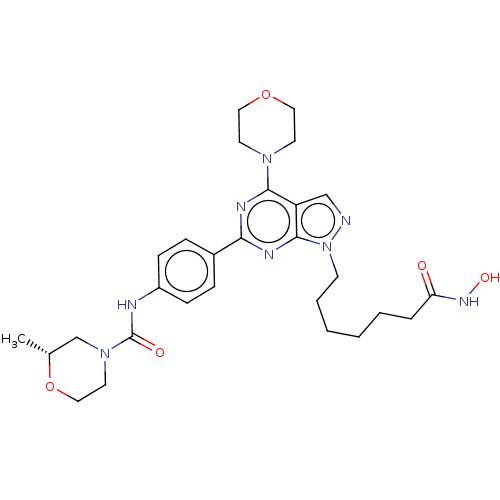
(CHEMBL4532398)Show SMILES C[C@@H]1CN(CCO1)C(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 |r| Show InChI InChI=1S/C28H38N8O5/c1-20-19-35(14-17-41-20)28(38)30-22-9-7-21(8-10-22)25-31-26(34-12-15-40-16-13-34)23-18-29-36(27(23)32-25)11-5-3-2-4-6-24(37)33-39/h7-10,18,20,39H,2-6,11-17,19H2,1H3,(H,30,38)(H,33,37)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length HDAC1 (unknown origin) using Ac-peptide-AMC as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543637
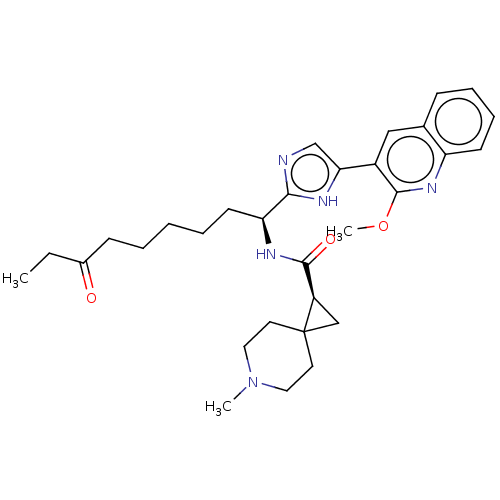
(CHEMBL4642518)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r| Show InChI InChI=1S/C31H41N5O3/c1-4-22(37)11-6-5-7-13-26(34-29(38)24-19-31(24)14-16-36(2)17-15-31)28-32-20-27(33-28)23-18-21-10-8-9-12-25(21)35-30(23)39-3/h8-10,12,18,20,24,26H,4-7,11,13-17,19H2,1-3H3,(H,32,33)(H,34,38)/t24-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50530002

(CHEMBL4532398)Show SMILES C[C@@H]1CN(CCO1)C(=O)Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 |r| Show InChI InChI=1S/C28H38N8O5/c1-20-19-35(14-17-41-20)28(38)30-22-9-7-21(8-10-22)25-31-26(34-12-15-40-16-13-34)23-18-29-36(27(23)32-25)11-5-3-2-4-6-24(37)33-39/h7-10,18,20,39H,2-6,11-17,19H2,1H3,(H,30,38)(H,33,37)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length HDAC1 (unknown origin) using Ac-peptide-AMC as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323704
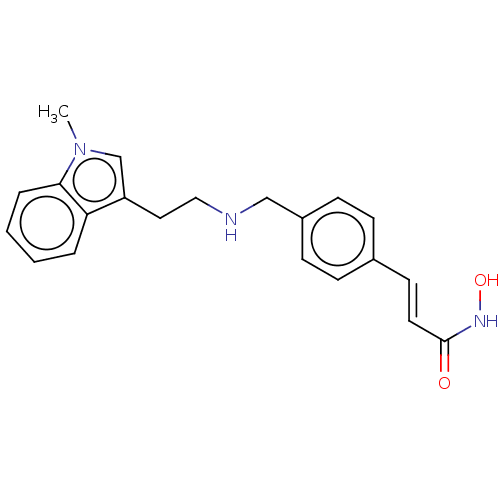
(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50544227
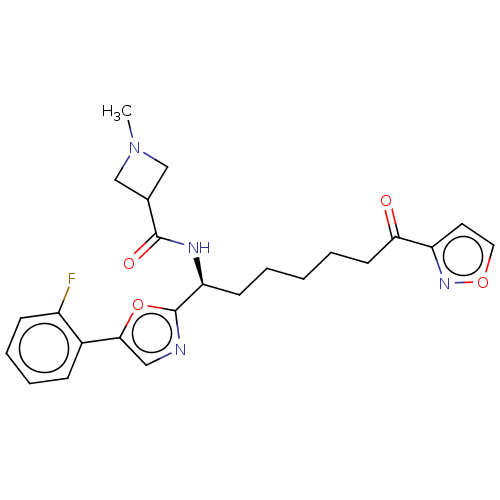
(CHEMBL4634501)Show SMILES CN1CC(C1)C(=O)N[C@@H](CCCCCC(=O)c1ccon1)c1ncc(o1)-c1ccccc1F |r| Show InChI InChI=1S/C24H27FN4O4/c1-29-14-16(15-29)23(31)27-20(9-3-2-4-10-21(30)19-11-12-32-28-19)24-26-13-22(33-24)17-7-5-6-8-18(17)25/h5-8,11-13,16,20H,2-4,9-10,14-15H2,1H3,(H,27,31)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of full length human recombinant FLAG-tagged HDAC1 expressed in HEK293F cells using FLUOR DE LYS as substrate preincubated for 3 hrs follo... |
ACS Med Chem Lett 11: 1476-1483 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00302
BindingDB Entry DOI: 10.7270/Q2RV0S85 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC1 using pan-HDAC substrate incubated for 3 hrs by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50552755
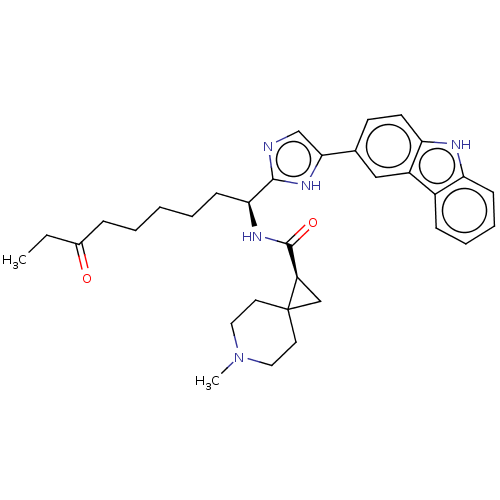
(CHEMBL4780309)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2[nH]c3ccccc3c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using compound purified by traditional preparative HPLC work-flow |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00596
BindingDB Entry DOI: 10.7270/Q2HD809G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50449386
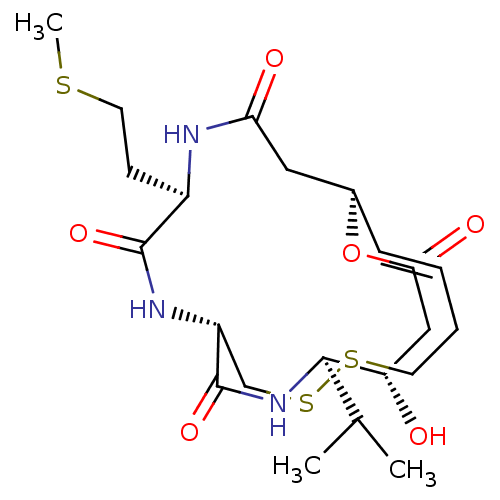
(CHEMBL3126833)Show SMILES CSCC[C@H]1NC(=O)C[C@@H]2OC(=O)C[C@H](O)[C@H](NC(=O)[C@@H](CSSCC\C=C\2)NC1=O)C(C)C |r,t:26| Show InChI InChI=1S/C22H35N3O6S3/c1-13(2)20-17(26)11-19(28)31-14-6-4-5-8-33-34-12-16(22(30)25-20)24-21(29)15(7-9-32-3)23-18(27)10-14/h4,6,13-17,20,26H,5,7-12H2,1-3H3,(H,23,27)(H,24,29)(H,25,30)/b6-4+/t14-,15-,16-,17+,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055512
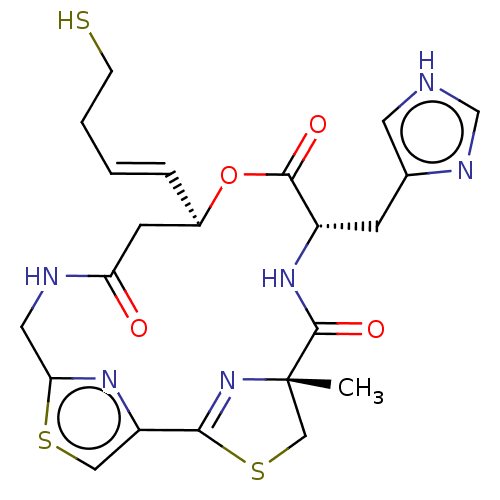
(CHEMBL3317812)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3c[nH]cn3)NC2=O)\C=C\CCS)n1 |r,c:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19151
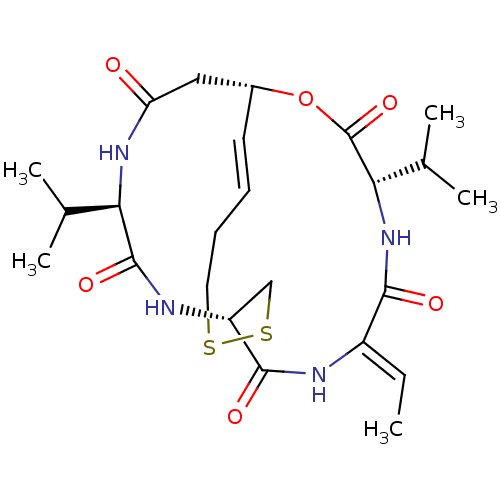
((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC1 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50386447

(CHEMBL2047701)Show InChI InChI=1S/C11H12Br2N2O3S/c12-7-3-6(4-8(13)10(7)16)5-9(15-18)11(17)14-1-2-19/h3-4,9,16,19H,1-2,5H2,(H,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... |
J Med Chem 55: 1731-50 (2012)
Article DOI: 10.1021/jm2016182
BindingDB Entry DOI: 10.7270/Q22F7PHC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323704

(US10188756, Compound CN107)Show InChI InChI=1S/C21H23N3O2/c1-24-15-18(19-4-2-3-5-20(19)24)12-13-22-14-17-8-6-16(7-9-17)10-11-21(25)23-26/h2-11,15,22,26H,12-14H2,1H3,(H,23,25)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation
US Patent
| Assay Description
All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... |
US Patent US10188756 (2019)
BindingDB Entry DOI: 10.7270/Q20Z75CX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568214

(CHEMBL4860000)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H]2CC22CCN(CC2)CCCCCCCCn2ccc3cc(ccc3c2=O)-c2cnc1[nH]2 |r,wU:13.12,wD:9.8,(54.03,-3.3,;52.63,-2.65,;51.39,-3.55,;51.52,-5.08,;49.99,-2.91,;48.73,-3.8,;47.34,-3.16,;46.08,-4.04,;44.69,-3.4,;43.44,-4.3,;43.59,-5.82,;44.98,-6.47,;46.23,-5.57,;45.14,-7.99,;46.02,-9.24,;44.49,-9.38,;43.11,-8.59,;41.76,-9.38,;41.75,-10.96,;43.12,-11.75,;44.51,-10.96,;40.36,-11.76,;38.97,-10.95,;37.58,-11.75,;36.09,-10.67,;34.26,-10.81,;32.5,-6.91,;30.88,-5.99,;31.12,-4.14,;32.53,-3.43,;33.01,-1.95,;34.54,-1.65,;35.57,-2.81,;37.06,-2.53,;38.07,-3.68,;37.59,-5.14,;36.08,-5.44,;35.07,-4.29,;33.55,-4.59,;33.04,-6.11,;39.58,-3.38,;40.22,-1.98,;41.75,-2.16,;42.05,-3.66,;40.71,-4.41,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128168
BindingDB Entry DOI: 10.7270/Q2QR51V7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50530003
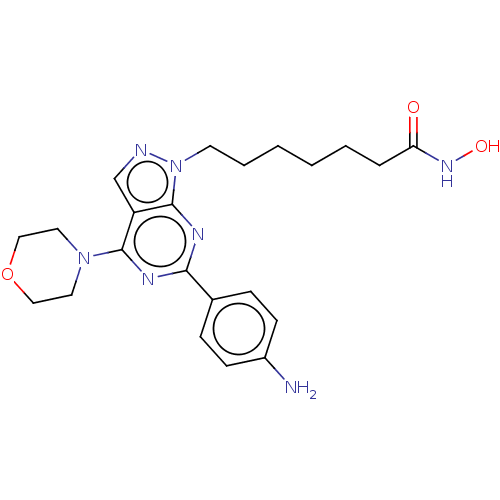
(CHEMBL4552057)Show SMILES Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 Show InChI InChI=1S/C22H29N7O3/c23-17-8-6-16(7-9-17)20-25-21(28-11-13-32-14-12-28)18-15-24-29(22(18)26-20)10-4-2-1-3-5-19(30)27-31/h6-9,15,31H,1-5,10-14,23H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length HDAC1 (unknown origin) using Ac-peptide-AMC as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055513
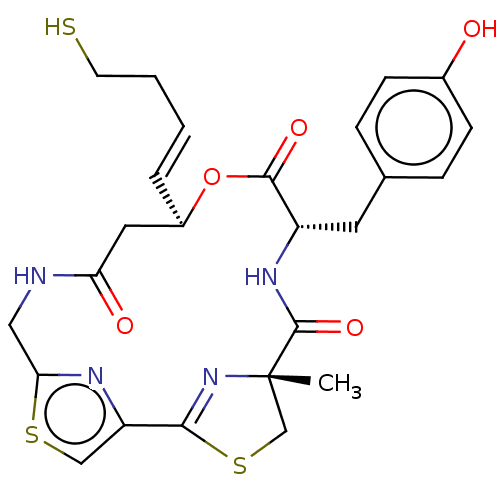
(CHEMBL3317811)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O5S3/c1-25-14-37-22(29-25)19-13-36-21(27-19)12-26-20(31)11-17(4-2-3-9-35)34-23(32)18(28-24(25)33)10-15-5-7-16(30)8-6-15/h2,4-8,13,17-18,30,35H,3,9-12,14H2,1H3,(H,26,31)(H,28,33)/b4-2+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50530003

(CHEMBL4552057)Show SMILES Nc1ccc(cc1)-c1nc(N2CCOCC2)c2cnn(CCCCCCC(=O)NO)c2n1 Show InChI InChI=1S/C22H29N7O3/c23-17-8-6-16(7-9-17)20-25-21(28-11-13-32-14-12-28)18-15-24-29(22(18)26-20)10-4-2-1-3-5-19(30)27-31/h6-9,15,31H,1-5,10-14,23H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length HDAC1 (unknown origin) using Ac-peptide-AMC as substrate preincubated for 15 mins followed by substrate additio... |
J Med Chem 62: 1577-1592 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01825
BindingDB Entry DOI: 10.7270/Q20868S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543647

(CHEMBL4637357)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C32H43N5O3/c1-4-23(38)12-7-6-8-14-27(35-30(39)25-20-32(25)15-17-37(5-2)18-16-32)29-33-21-28(34-29)24-19-22-11-9-10-13-26(22)36-31(24)40-3/h9-11,13,19,21,25,27H,4-8,12,14-18,20H2,1-3H3,(H,33,34)(H,35,39)/t25-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length FLAG-tagged HDAC1 expressed in HEK293F cells using BML-KI104 as substrate in presence of SAHA as inhibito... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00462
BindingDB Entry DOI: 10.7270/Q2TH8RDR |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543647

(CHEMBL4637357)Show SMILES CCN1CCC2(C[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C32H43N5O3/c1-4-23(38)12-7-6-8-14-27(35-30(39)25-20-32(25)15-17-37(5-2)18-16-32)29-33-21-28(34-29)24-19-22-11-9-10-13-26(22)36-31(24)40-3/h9-11,13,19,21,25,27H,4-8,12,14-18,20H2,1-3H3,(H,33,34)(H,35,39)/t25-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | 20 |
DOUBLE RIDER MEDICINE CO., LTD.
US Patent
| Assay Description
Enzymatic activity was tested in 96-well or 384-well flat microwell plate by fluorescence detection and taking Ac-Lys-Tyr-Lys(Ac)-AMC as substrate. T... |
US Patent US9695181 (2017)
BindingDB Entry DOI: 10.7270/Q2RX9971 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543643
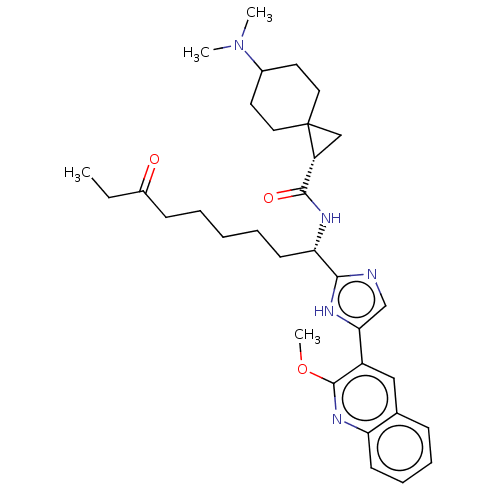
(CHEMBL4642023)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCC(CC1)N(C)C)c1ncc([nH]1)-c1cc2ccccc2nc1OC |r,wD:9.9,13.12,(21.19,-2.07,;19.64,-2.07,;18.88,-3.41,;19.65,-4.74,;17.34,-3.41,;16.57,-4.75,;15.03,-4.75,;14.26,-6.09,;12.72,-6.09,;11.95,-7.43,;12.72,-8.76,;14.27,-8.76,;15.03,-7.42,;15.03,-10.08,;15.04,-11.61,;16.36,-10.84,;17.12,-12.17,;18.64,-12.17,;19.41,-10.85,;18.65,-9.53,;17.12,-9.52,;20.94,-10.86,;21.7,-12.19,;21.72,-9.54,;10.41,-7.43,;9.64,-6.1,;8.13,-6.43,;7.98,-7.96,;9.39,-8.57,;6.66,-8.74,;5.32,-7.97,;3.99,-8.75,;2.65,-7.98,;1.32,-8.76,;1.32,-10.3,;2.66,-11.07,;3.99,-10.3,;5.33,-11.06,;6.66,-10.29,;8,-11.05,;8.01,-12.59,)| Show InChI InChI=1S/C33H45N5O3/c1-5-24(39)12-7-6-8-14-28(36-31(40)26-20-33(26)17-15-23(16-18-33)38(2)3)30-34-21-29(35-30)25-19-22-11-9-10-13-27(22)37-32(25)41-4/h9-11,13,19,21,23,26,28H,5-8,12,14-18,20H2,1-4H3,(H,34,35)(H,36,40)/t23?,26-,28+,33?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50354088
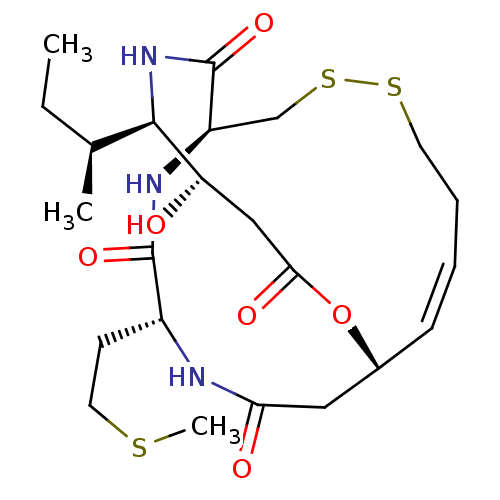
(CHEMBL1836142)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](CCSC)C(=O)N2)OC(=O)C[C@@H]1O |r,t:14| Show InChI InChI=1S/C23H37N3O6S3/c1-4-14(2)21-18(27)12-20(29)32-15-7-5-6-9-34-35-13-17(23(31)26-21)25-22(30)16(8-10-33-3)24-19(28)11-15/h5,7,14-18,21,27H,4,6,8-13H2,1-3H3,(H,24,28)(H,25,30)(H,26,31)/b7-5+/t14-,15+,16+,17+,18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Milwaukee
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 1 hr by luminescence assay |
Medchemcomm 3: 976-981 (2012)
Article DOI: 10.1039/c2md20024d
BindingDB Entry DOI: 10.7270/Q21G0Q7K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606543
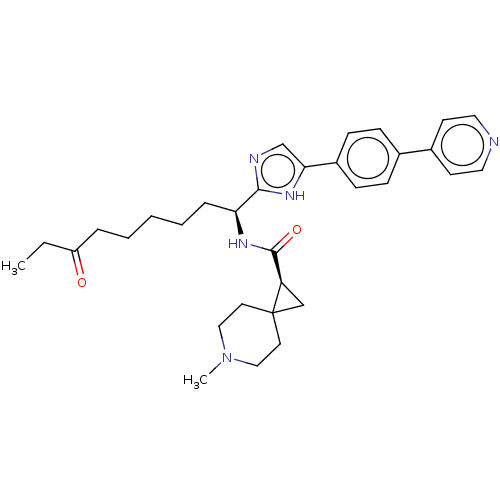
(CHEMBL5218926)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc(cc1)-c1ccncc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50055514
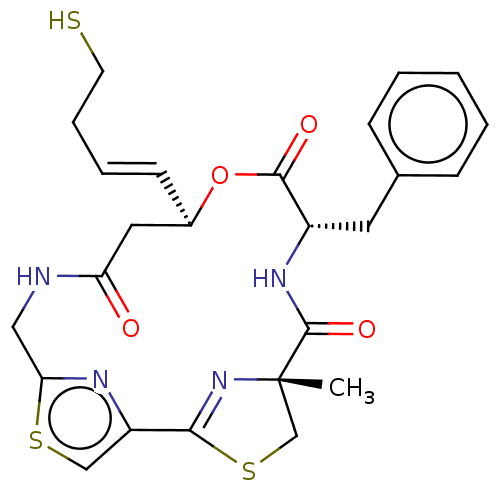
(CHEMBL3317810)Show SMILES C[C@@]12CSC(=N1)c1csc(CNC(=O)C[C@H](OC(=O)[C@H](Cc3ccccc3)NC2=O)\C=C\CCS)n1 |r,c:4| Show InChI InChI=1S/C25H28N4O4S3/c1-25-15-36-22(29-25)19-14-35-21(27-19)13-26-20(30)12-17(9-5-6-10-34)33-23(31)18(28-24(25)32)11-16-7-3-2-4-8-16/h2-5,7-9,14,17-18,34H,6,10-13,15H2,1H3,(H,26,30)(H,28,32)/b9-5+/t17-,18+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) incubated at 37 degC for 30 mins |
Bioorg Med Chem Lett 24: 3728-31 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.006
BindingDB Entry DOI: 10.7270/Q25Q4XRZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50543640
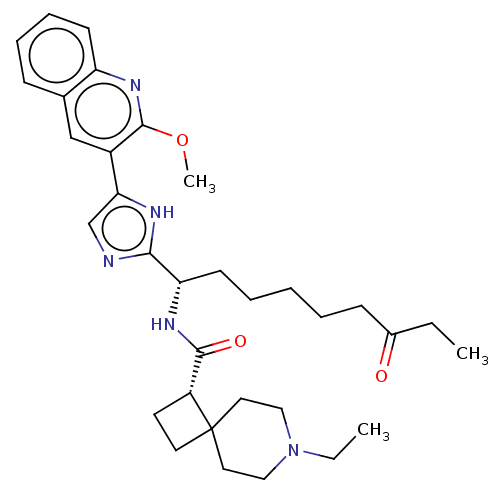
(CHEMBL4638264)Show SMILES CCN1CCC2(CC[C@@H]2C(=O)N[C@@H](CCCCCC(=O)CC)c2ncc([nH]2)-c2cc3ccccc3nc2OC)CC1 |r| Show InChI InChI=1S/C33H45N5O3/c1-4-24(39)12-7-6-8-14-28(36-31(40)26-15-16-33(26)17-19-38(5-2)20-18-33)30-34-22-29(35-30)25-21-23-11-9-10-13-27(23)37-32(25)41-3/h9-11,13,21-22,26,28H,4-8,12,14-20H2,1-3H3,(H,34,35)(H,36,40)/t26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127197
BindingDB Entry DOI: 10.7270/Q2CZ3BQC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50568207

(CHEMBL4856631)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2c(ccn(C)c2=O)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM323709
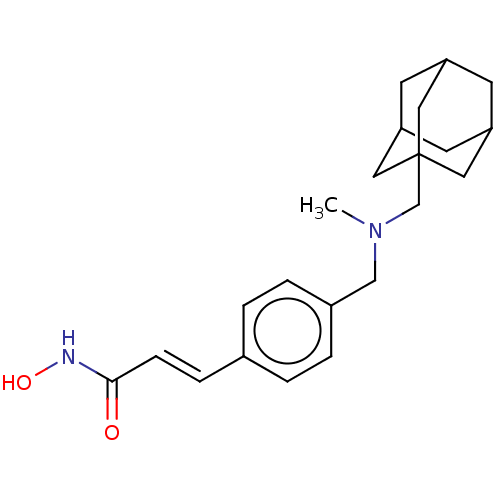
(US10188756, Compound CN133 | US11207431, Martinost...)Show SMILES CN(Cc1ccc(\C=C\C(=O)NO)cc1)CC12CC3CC(CC(C3)C1)C2 |TLB:19:20:24:18.23.17,23:18:25:24.22.21,23:22:25:18.19.17,THB:19:18:24:25.20.21| Show InChI InChI=1S/C22H30N2O2/c1-24(14-17-4-2-16(3-5-17)6-7-21(25)23-26)15-22-11-18-8-19(12-22)10-20(9-18)13-22/h2-7,18-20,26H,8-15H2,1H3,(H,23,25)/b7-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The General Hospital Corporation
US Patent
| Assay Description
All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a... |
US Patent US10188756 (2019)
BindingDB Entry DOI: 10.7270/Q20Z75CX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50606548
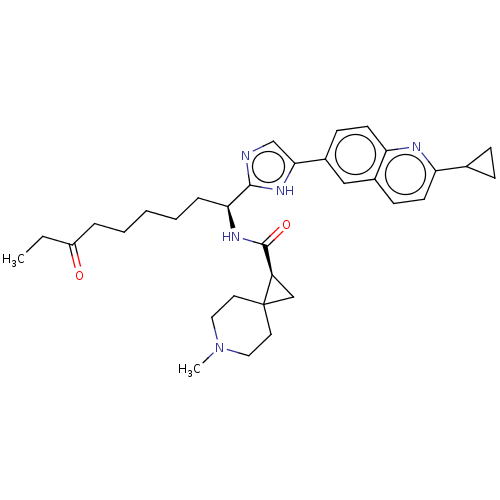
(CHEMBL5218918)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)[C@H]1CC11CCN(C)CC1)c1ncc([nH]1)-c1ccc2nc(ccc2c1)C1CC1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02150
BindingDB Entry DOI: 10.7270/Q2N58RGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data