 Found 2088 hits of ic50 for UniProtKB: P43235
Found 2088 hits of ic50 for UniProtKB: P43235 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM19737
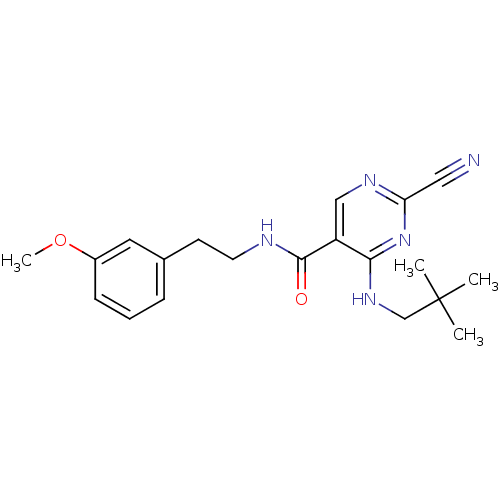
(2-Cyano-pyrimidine, 17b | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1cccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)c1 Show InChI InChI=1S/C20H25N5O2/c1-20(2,3)13-24-18-16(12-23-17(11-21)25-18)19(26)22-9-8-14-6-5-7-15(10-14)27-4/h5-7,10,12H,8-9,13H2,1-4H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19736
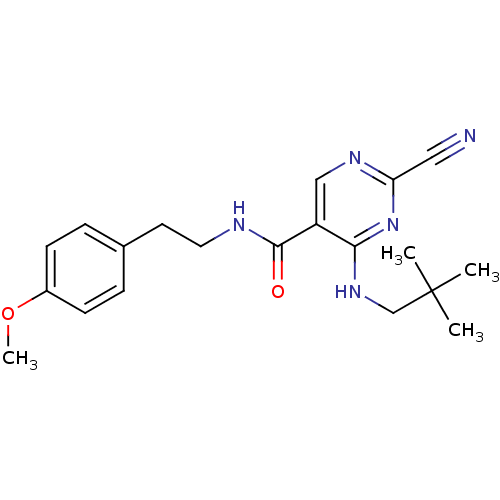
(2-Cyano-pyrimidine, 17a | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1 Show InChI InChI=1S/C20H25N5O2/c1-20(2,3)13-24-18-16(12-23-17(11-21)25-18)19(26)22-10-9-14-5-7-15(27-4)8-6-14/h5-8,12H,9-10,13H2,1-4H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19744
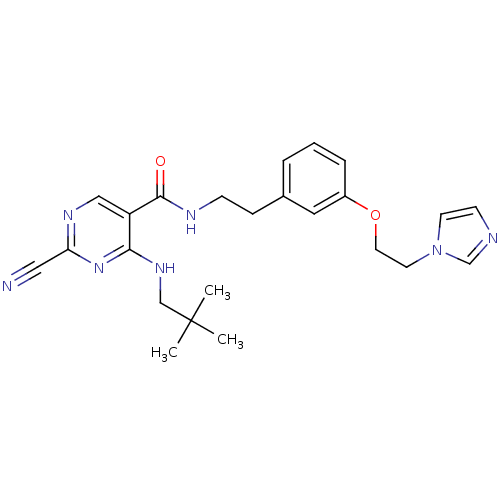
(2-Cyano-pyrimidine, 17i | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CC(C)(C)CNc1nc(ncc1C(=O)NCCc1cccc(OCCn2ccnc2)c1)C#N Show InChI InChI=1S/C24H29N7O2/c1-24(2,3)16-29-22-20(15-28-21(14-25)30-22)23(32)27-8-7-18-5-4-6-19(13-18)33-12-11-31-10-9-26-17-31/h4-6,9-10,13,15,17H,7-8,11-12,16H2,1-3H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19743
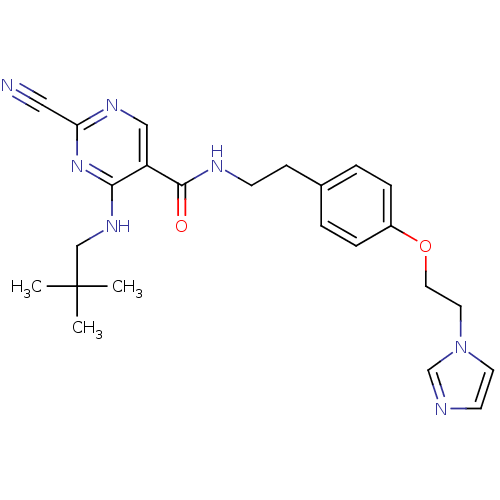
(2-Cyano-pyrimidine, 17h | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CC(C)(C)CNc1nc(ncc1C(=O)NCCc1ccc(OCCn2ccnc2)cc1)C#N Show InChI InChI=1S/C24H29N7O2/c1-24(2,3)16-29-22-20(15-28-21(14-25)30-22)23(32)27-9-8-18-4-6-19(7-5-18)33-13-12-31-11-10-26-17-31/h4-7,10-11,15,17H,8-9,12-13,16H2,1-3H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19733

(2-Cyano-pyrimidine, 16c | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C21H25N5O2/c1-28-17-9-5-6-15(12-17)10-11-23-21(27)18-14-24-19(13-22)26-20(18)25-16-7-3-2-4-8-16/h5-6,9,12,14,16H,2-4,7-8,10-11H2,1H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19731

(2-Cyano-pyrimidine, 16a | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C20H23N5O/c21-13-18-23-14-17(19(25-18)24-16-9-5-2-6-10-16)20(26)22-12-11-15-7-3-1-4-8-15/h1,3-4,7-8,14,16H,2,5-6,9-12H2,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19741
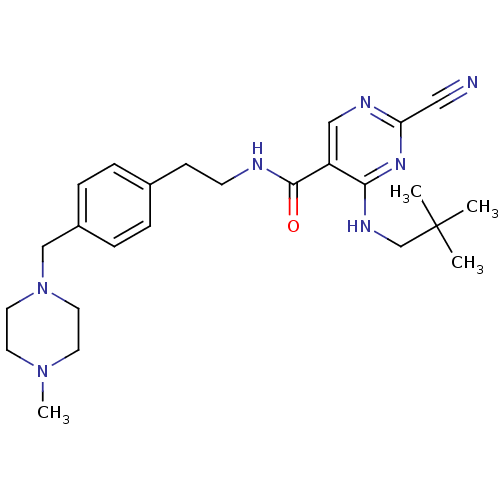
(2-Cyano-pyrimidine, 17f | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCN(Cc2ccc(CCNC(=O)c3cnc(nc3NCC(C)(C)C)C#N)cc2)CC1 Show InChI InChI=1S/C25H35N7O/c1-25(2,3)18-29-23-21(16-28-22(15-26)30-23)24(33)27-10-9-19-5-7-20(8-6-19)17-32-13-11-31(4)12-14-32/h5-8,16H,9-14,17-18H2,1-4H3,(H,27,33)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19740
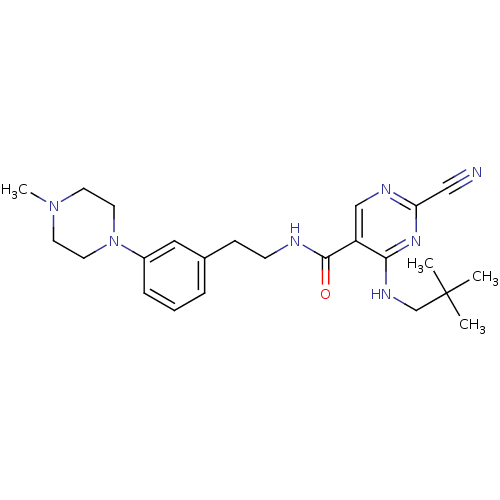
(2-Cyano-pyrimidine, 17e | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCN(CC1)c1cccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)c1 Show InChI InChI=1S/C24H33N7O/c1-24(2,3)17-28-22-20(16-27-21(15-25)29-22)23(32)26-9-8-18-6-5-7-19(14-18)31-12-10-30(4)11-13-31/h5-7,14,16H,8-13,17H2,1-4H3,(H,26,32)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19742
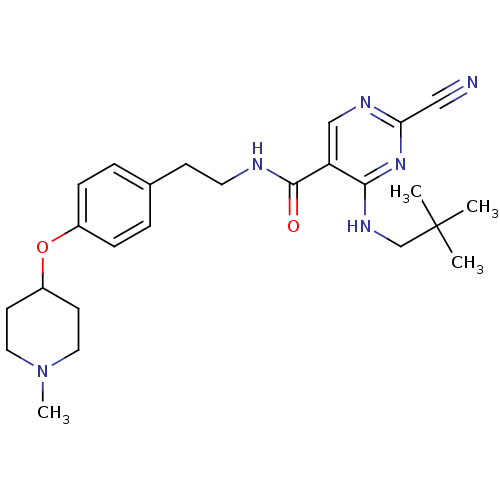
(2-Cyano-pyrimidine, 17g | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCC(CC1)Oc1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1 Show InChI InChI=1S/C25H34N6O2/c1-25(2,3)17-29-23-21(16-28-22(15-26)30-23)24(32)27-12-9-18-5-7-19(8-6-18)33-20-10-13-31(4)14-11-20/h5-8,16,20H,9-14,17H2,1-4H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50251429
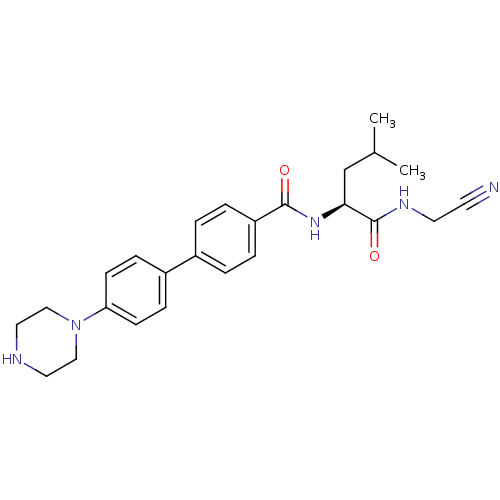
(4'-Piperazin-1-yl-biphenyl-4-carboxylic acid [(S)-...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N |r| Show InChI InChI=1S/C25H31N5O2/c1-18(2)17-23(25(32)28-12-11-26)29-24(31)21-5-3-19(4-6-21)20-7-9-22(10-8-20)30-15-13-27-14-16-30/h3-10,18,23,27H,12-17H2,1-2H3,(H,28,32)(H,29,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19732

(2-Cyano-pyrimidine, 16b | 2-cyano-4-(cyclohexylami...)Show InChI InChI=1S/C21H25N5O2/c1-28-17-9-7-15(8-10-17)11-12-23-21(27)18-14-24-19(13-22)26-20(18)25-16-5-3-2-4-6-16/h7-10,14,16H,2-6,11-12H2,1H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19739
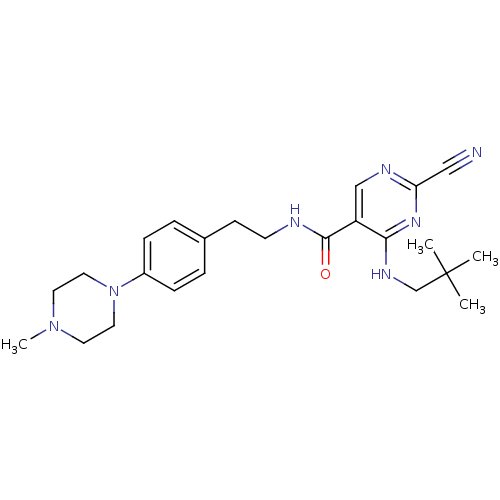
(2-Cyano-pyrimidine, 17d | 2-cyano-4-[(2,2-dimethyl...)Show SMILES CN1CCN(CC1)c1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1 Show InChI InChI=1S/C24H33N7O/c1-24(2,3)17-28-22-20(16-27-21(15-25)29-22)23(32)26-10-9-18-5-7-19(8-6-18)31-13-11-30(4)12-14-31/h5-8,16H,9-14,17H2,1-4H3,(H,26,32)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50169493

((S)-3,3-dimethyl-1-(4-(4-(trifluoromethyl)phenyl)-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1cnc(c1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-19(23(37)24(38)34-22-12-13-32-35-22)33-25(39)40-21(26(2,3)4)15-36-14-20(31-16-36)17-8-10-18(11-9-17)27(28,29)30/h8-14,16,19,21H,5-7,15H2,1-4H3,(H,33,39)(H2,32,34,35,38)/t19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50169495
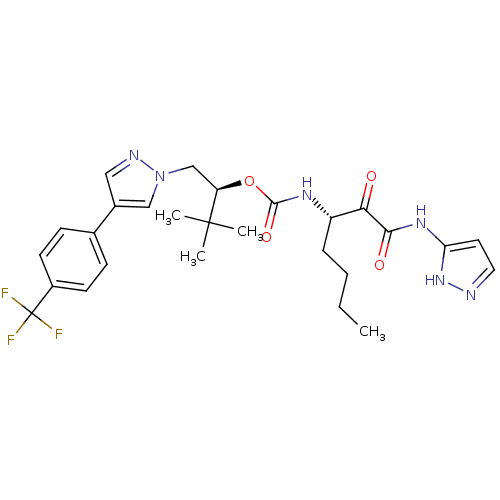
((S)-3,3-dimethyl-1-(4-(4-(trifluoromethyl)phenyl)-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1cc(cn1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-20(23(37)24(38)34-22-12-13-31-35-22)33-25(39)40-21(26(2,3)4)16-36-15-18(14-32-36)17-8-10-19(11-9-17)27(28,29)30/h8-15,20-21H,5-7,16H2,1-4H3,(H,33,39)(H2,31,34,35,38)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0257 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50169482
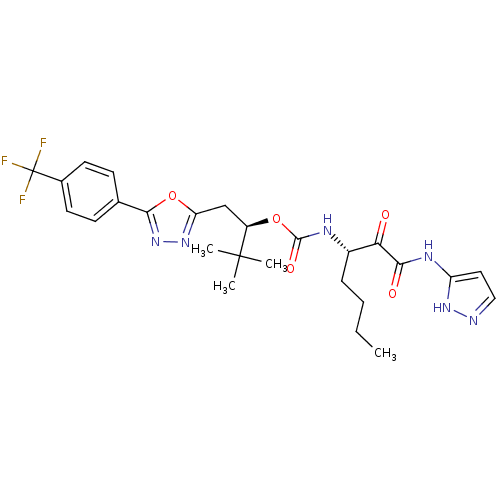
((R)-3,3-dimethyl-1-(5-(4-(trifluoromethyl)phenyl)-...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cc1nnc(o1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 Show InChI InChI=1S/C26H31F3N6O5/c1-5-6-7-17(21(36)22(37)32-19-12-13-30-33-19)31-24(38)39-18(25(2,3)4)14-20-34-35-23(40-20)15-8-10-16(11-9-15)26(27,28)29/h8-13,17-18H,5-7,14H2,1-4H3,(H,31,38)(H2,30,32,33,37)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0288 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19745

(2-Cyano-pyrimidine, 17j | 2-cyano-4-[(2,2-dimethyl...)Show SMILES COc1ccc(CCNC(=O)c2cnc(nc2NCC(C)(C)C)C#N)cc1OCCn1ccnc1 Show InChI InChI=1S/C25H31N7O3/c1-25(2,3)16-30-23-19(15-29-22(14-26)31-23)24(33)28-8-7-18-5-6-20(34-4)21(13-18)35-12-11-32-10-9-27-17-32/h5-6,9-10,13,15,17H,7-8,11-12,16H2,1-4H3,(H,28,33)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19735

(2-Cyano-pyrimidine, 16e | 2-cyano-4-(cyclohexylami...)Show SMILES CN1CCN(CC1)c1ccc(CCNC(=O)c2cnc(nc2NC2CCCCC2)C#N)cc1 Show InChI InChI=1S/C25H33N7O/c1-31-13-15-32(16-14-31)21-9-7-19(8-10-21)11-12-27-25(33)22-18-28-23(17-26)30-24(22)29-20-5-3-2-4-6-20/h7-10,18,20H,2-6,11-16H2,1H3,(H,27,33)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis Pharmaceuticals
| Assay Description
The substrate hydrolysis with or without inhibitor was monitored at an excitation wavelength of 360nm and an emission wavelength of 460 nm on a fluor... |
J Med Chem 50: 591-4 (2007)
Article DOI: 10.1021/jm0613525
BindingDB Entry DOI: 10.7270/Q22805X5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163831
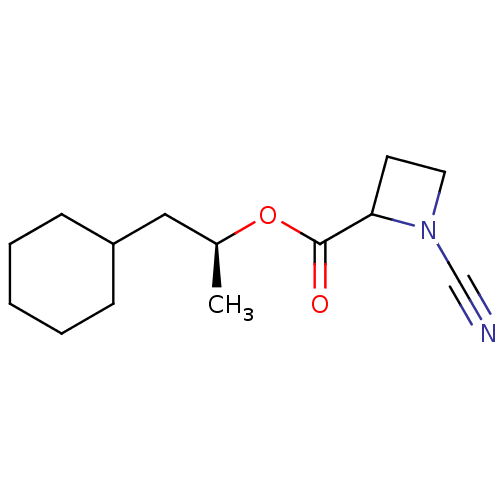
((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19783
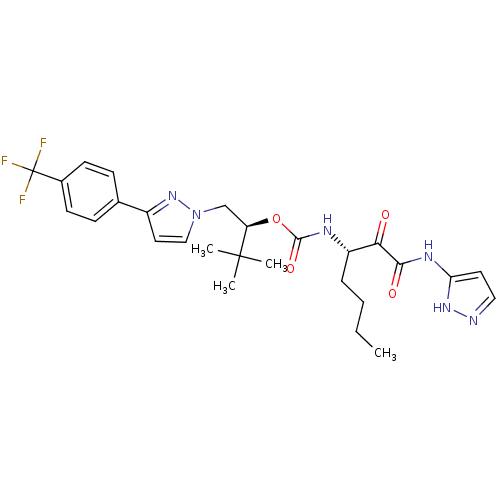
((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 |r| Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-20(23(37)24(38)33-22-12-14-31-34-22)32-25(39)40-21(26(2,3)4)16-36-15-13-19(35-36)17-8-10-18(11-9-17)27(28,29)30/h8-15,20-21H,5-7,16H2,1-4H3,(H,32,39)(H2,31,33,34,38)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK
| Assay Description
Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut... |
Bioorg Med Chem Lett 17: 22-7 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.102
BindingDB Entry DOI: 10.7270/Q2NZ85XC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19783

((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cn1ccc(n1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)Nc1ccn[nH]1 |r| Show InChI InChI=1S/C27H33F3N6O4/c1-5-6-7-20(23(37)24(38)33-22-12-14-31-34-22)32-25(39)40-21(26(2,3)4)16-36-15-13-19(35-36)17-8-10-18(11-9-17)27(28,29)30/h8-15,20-21H,5-7,16H2,1-4H3,(H,32,39)(H2,31,33,34,38)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0724 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084655
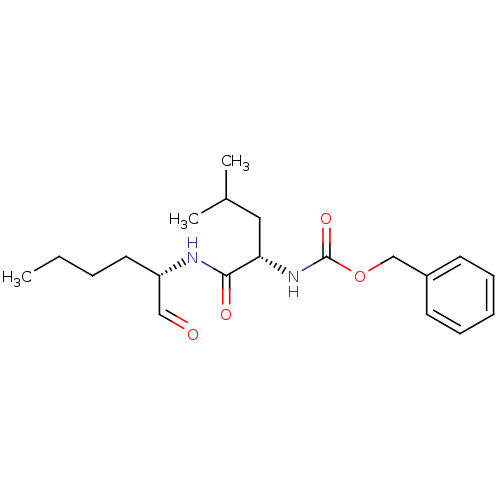
(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibiory activity against recombinant human cathepsin K |
Bioorg Med Chem Lett 14: 275-8 (2003)
BindingDB Entry DOI: 10.7270/Q2GM86PN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461260
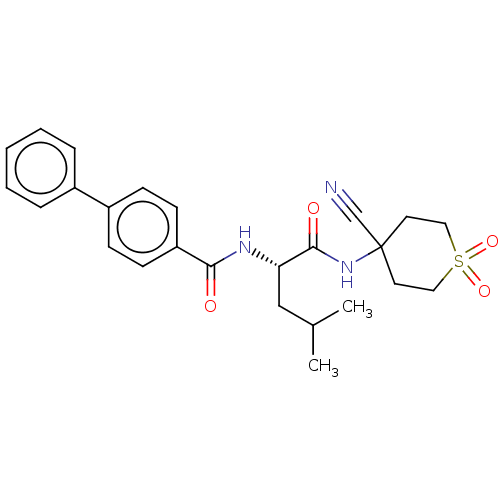
(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50084655

(CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-(...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C20H30N2O4/c1-4-5-11-17(13-23)21-19(24)18(12-15(2)3)22-20(25)26-14-16-9-7-6-8-10-16/h6-10,13,15,17-18H,4-5,11-12,14H2,1-3H3,(H,21,24)(H,22,25)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cathepsin K |
Bioorg Med Chem Lett 14: 719-22 (2004)
BindingDB Entry DOI: 10.7270/Q2QV3KX1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50169488
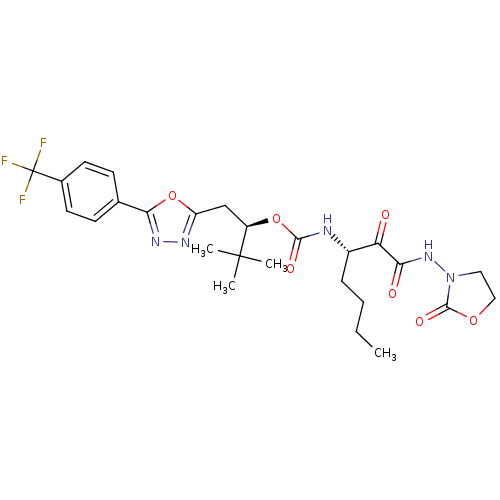
((1R)-2,2-DIMETHYL-1-({5-[4-(TRIFLUOROMETHYL)PHENYL...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cc1nnc(o1)-c1ccc(cc1)C(F)(F)F)C(C)(C)C)C(=O)C(=O)NN1CCOC1=O Show InChI InChI=1S/C26H32F3N5O7/c1-5-6-7-17(20(35)21(36)33-34-12-13-39-24(34)38)30-23(37)40-18(25(2,3)4)14-19-31-32-22(41-19)15-8-10-16(11-9-15)26(27,28)29/h8-11,17-18H,5-7,12-14H2,1-4H3,(H,30,37)(H,33,36)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148310

(((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-p...)Show InChI InChI=1S/C17H25NO3/c1-3-5-11-15(13-19)18-17(20)21-16(4-2)12-14-9-7-6-8-10-14/h6-10,13,15-16H,3-5,11-12H2,1-2H3,(H,18,20)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148310

(((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-p...)Show InChI InChI=1S/C17H25NO3/c1-3-5-11-15(13-19)18-17(20)21-16(4-2)12-14-9-7-6-8-10-14/h6-10,13,15-16H,3-5,11-12H2,1-2H3,(H,18,20)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human cathepsin K determined in a fluorescence assay using 10 microM Cbz-Phe-Arg-AMC as substrate |
Bioorg Med Chem Lett 14: 3425-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.04.084
BindingDB Entry DOI: 10.7270/Q2SF2VMD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255925
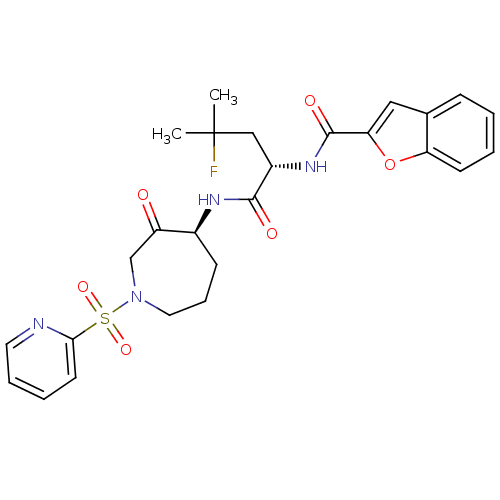
(CHEMBL474438 | N-((S)-4-fluoro-4-methyl-1-oxo-1-((...)Show SMILES CC(C)(F)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H29FN4O6S/c1-26(2,27)15-19(30-25(34)22-14-17-8-3-4-10-21(17)37-22)24(33)29-18-9-7-13-31(16-20(18)32)38(35,36)23-11-5-6-12-28-23/h3-6,8,10-12,14,18-19H,7,9,13,15-16H2,1-2H3,(H,29,33)(H,30,34)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 19: 675-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.053
BindingDB Entry DOI: 10.7270/Q21V5DVM |
More data for this
Ligand-Target Pair | |
Cathepsin K
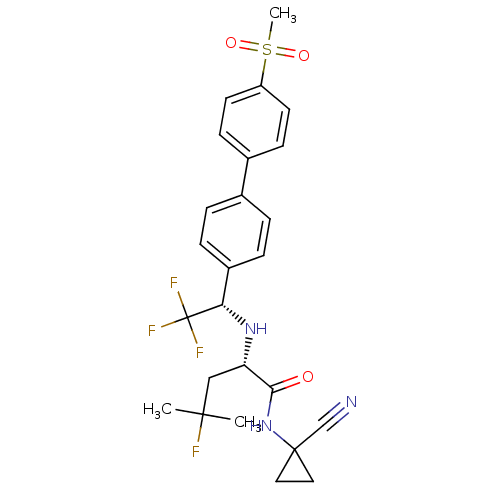
(Homo sapiens (Human)) | BDBM50255753
(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 53: 4332-53 (2010)
Article DOI: 10.1021/jm9018756
BindingDB Entry DOI: 10.7270/Q21R6QPF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489
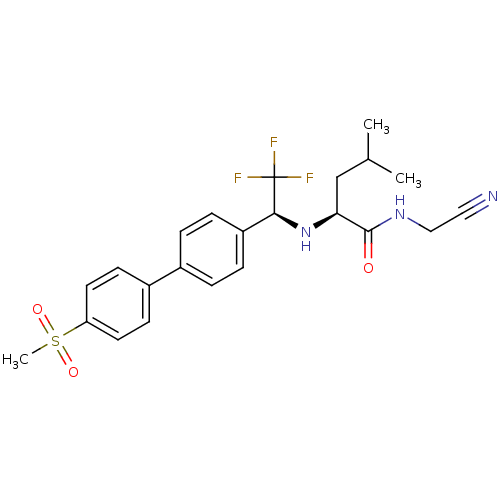
((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 21: 920-3 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.070
BindingDB Entry DOI: 10.7270/Q2Z89CQF |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
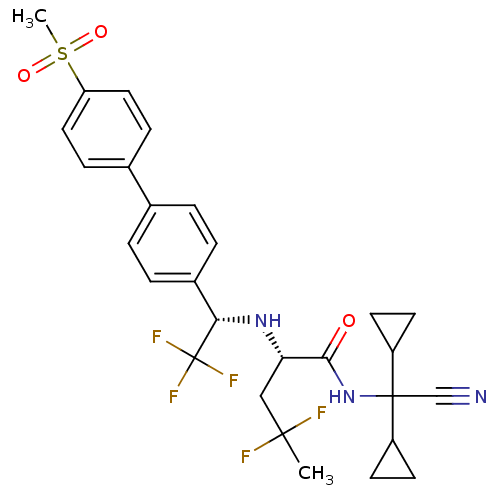
(Homo sapiens (Human)) | BDBM50233032
((S)-4,4-difluoro-2-[(S)-2,2,2-trifluoro-1-(4'-meth...)Show SMILES CC(F)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC(C#N)(C1CC1)C1CC1 Show InChI InChI=1S/C28H30F5N3O3S/c1-26(29,30)15-23(25(37)36-27(16-34,20-9-10-20)21-11-12-21)35-24(28(31,32)33)19-5-3-17(4-6-19)18-7-13-22(14-8-18)40(2,38)39/h3-8,13-14,20-21,23-24,35H,9-12,15H2,1-2H3,(H,36,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 18: 923-8 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.047
BindingDB Entry DOI: 10.7270/Q21J9BM2 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50150528

((S)-4-Methyl-2-[4-(4-piperazin-1-yl-phenyl)-thioph...)Show SMILES CC(C)C[C@H](Nc1cscc1-c1ccc(cc1)N1CCNCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5OS/c1-16(2)13-20(22(28)25-8-7-23)26-21-15-29-14-19(21)17-3-5-18(6-4-17)27-11-9-24-10-12-27/h3-6,14-16,20,24,26H,8-13H2,1-2H3,(H,25,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K |
Bioorg Med Chem Lett 14: 4291-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.087
BindingDB Entry DOI: 10.7270/Q2765DS1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50214543

((S)-4-methyl-2-[(S)-2,2,2-trifluoro-1-(4'-methanes...)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C#N Show InChI InChI=1S/C26H32F3N3O3S2/c1-17(2)15-23(25(33)31-21(16-30)13-14-36-3)32-24(26(27,28)29)20-7-5-18(6-8-20)19-9-11-22(12-10-19)37(4,34)35/h5-12,17,21,23-24,32H,13-15H2,1-4H3,(H,31,33)/t21-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 17: 4328-32 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.024
BindingDB Entry DOI: 10.7270/Q2TQ618K |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19489

((2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifl...)Show SMILES CC(C)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NCC#N |r| Show InChI InChI=1S/C23H26F3N3O3S/c1-15(2)14-20(22(30)28-13-12-27)29-21(23(24,25)26)18-6-4-16(5-7-18)17-8-10-19(11-9-17)33(3,31)32/h4-11,15,20-21,29H,13-14H2,1-3H3,(H,28,30)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 19: 675-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.053
BindingDB Entry DOI: 10.7270/Q21V5DVM |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306304

((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C(C)(C)O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H31F4N3O2/c1-24(2,28)15-21(23(35)34-26(16-32)13-14-26)33-22(27(29,30)31)19-7-5-17(6-8-19)18-9-11-20(12-10-18)25(3,4)36/h5-12,21-22,33,36H,13-15H2,1-4H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306306
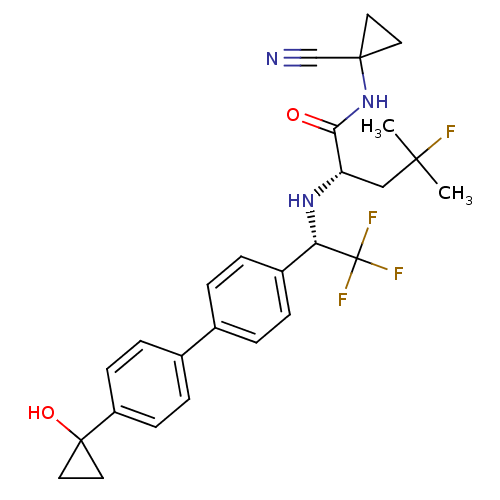
((S)-N-(1-cyanocyclopropyl)-4-fluoro-4-methyl-2-((S...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(O)CC1)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C27H29F4N3O2/c1-24(2,28)15-21(23(35)34-25(16-32)11-12-25)33-22(27(29,30)31)19-5-3-17(4-6-19)18-7-9-20(10-8-18)26(36)13-14-26/h3-10,21-22,33,36H,11-15H2,1-2H3,(H,34,35)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306307

((S)-2-((S)-1-(4'-((S)-1-amino-1-oxopropan-2-yl)bip...)Show SMILES C[C@H](C(N)=O)c1ccc(cc1)-c1ccc(cc1)[C@H](N[C@@H](CC(C)(C)F)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N4O2/c1-16(23(33)36)17-4-6-18(7-5-17)19-8-10-20(11-9-19)22(27(29,30)31)34-21(14-25(2,3)28)24(37)35-26(15-32)12-13-26/h4-11,16,21-22,34H,12-14H2,1-3H3,(H2,33,36)(H,35,37)/t16-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50306309

(1-(4'-((S)-1-((S)-1-(1-cyanocyclopropylamino)-4-fl...)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)C1(CC1)C(N)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C28H30F4N4O2/c1-25(2,29)15-21(23(37)36-26(16-33)11-12-26)35-22(28(30,31)32)19-5-3-17(4-6-19)18-7-9-20(10-8-18)27(13-14-27)24(34)38/h3-10,21-22,35H,11-15H2,1-2H3,(H2,34,38)(H,36,37)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50255753

(CHEMBL481611 | MK-0822 | Odanacatib)Show SMILES CC(C)(F)C[C@H](N[C@@H](c1ccc(cc1)-c1ccc(cc1)S(C)(=O)=O)C(F)(F)F)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of humanized rabbit cathepsin K |
Bioorg Med Chem Lett 20: 887-92 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.083
BindingDB Entry DOI: 10.7270/Q27D2V75 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138858
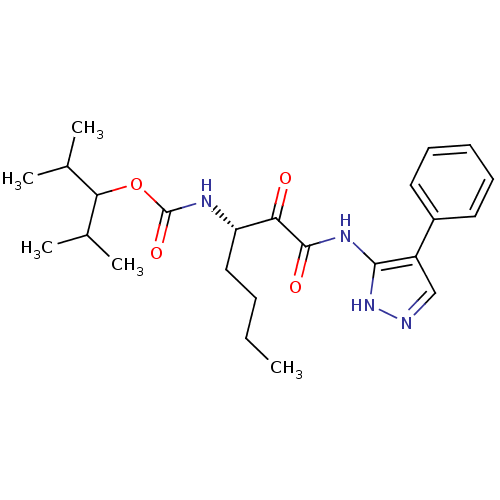
(CHEMBL154579 | [(S)-1-(4-Phenyl-1H-pyrazol-3-ylami...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1-c1ccccc1 Show InChI InChI=1S/C24H34N4O4/c1-6-7-13-19(26-24(31)32-21(15(2)3)16(4)5)20(29)23(30)27-22-18(14-25-28-22)17-11-9-8-10-12-17/h8-12,14-16,19,21H,6-7,13H2,1-5H3,(H,26,31)(H2,25,27,28,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50138853
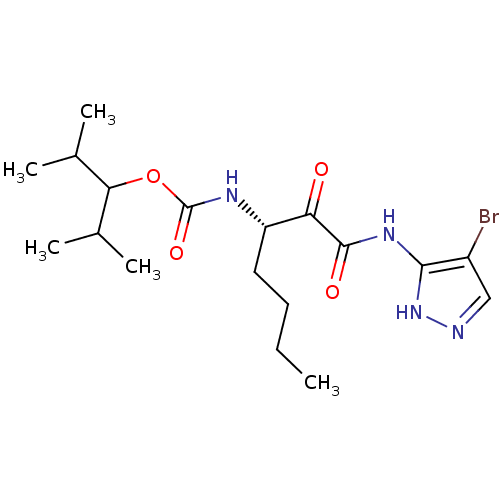
(CHEMBL157072 | [(S)-1-(4-Bromo-1H-pyrazol-3-ylamin...)Show SMILES CCCC[C@H](NC(=O)OC(C(C)C)C(C)C)C(=O)C(=O)Nc1[nH]ncc1Br Show InChI InChI=1S/C18H29BrN4O4/c1-6-7-8-13(21-18(26)27-15(10(2)3)11(4)5)14(24)17(25)22-16-12(19)9-20-23-16/h9-11,13,15H,6-8H2,1-5H3,(H,21,26)(H2,20,22,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% of cysteine protease cathepsin K of human |
J Med Chem 47: 588-99 (2004)
Article DOI: 10.1021/jm030373l
BindingDB Entry DOI: 10.7270/Q29S1QF7 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50169496
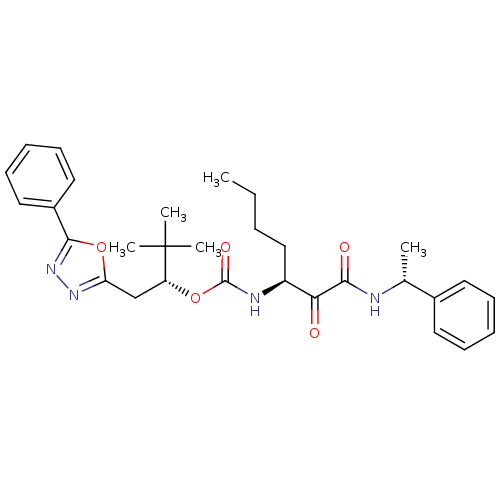
((R)-3,3-dimethyl-1-(5-phenyl-1,3,4-oxadiazol-2-yl)...)Show SMILES CCCC[C@H](NC(=O)O[C@H](Cc1nnc(o1)-c1ccccc1)C(C)(C)C)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C30H38N4O5/c1-6-7-18-23(26(35)27(36)31-20(2)21-14-10-8-11-15-21)32-29(37)38-24(30(3,4)5)19-25-33-34-28(39-25)22-16-12-9-13-17-22/h8-17,20,23-24H,6-7,18-19H2,1-5H3,(H,31,36)(H,32,37)/t20-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Eur J Med Chem 45: 667-81 (2010)
Article DOI: 10.1016/j.ejmech.2009.11.010
BindingDB Entry DOI: 10.7270/Q29C6ZP1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50426168

(CHEMBL2316303)Show SMILES CCCCC(NC(=O)C(CC(C)C)NC(=O)OCc1ccc(Br)cc1)C=O Show InChI InChI=1S/C20H29BrN2O4/c1-4-5-6-17(12-24)22-19(25)18(11-14(2)3)23-20(26)27-13-15-7-9-16(21)10-8-15/h7-10,12,14,17-18H,4-6,11,13H2,1-3H3,(H,22,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Animal Health Innovation GmbH
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K (unknown origin) |
J Med Chem 56: 1478-90 (2013)
Article DOI: 10.1021/jm3013932
BindingDB Entry DOI: 10.7270/Q2W09774 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data