 Found 248 hits of kd for UniProtKB: P48736
Found 248 hits of kd for UniProtKB: P48736 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192880
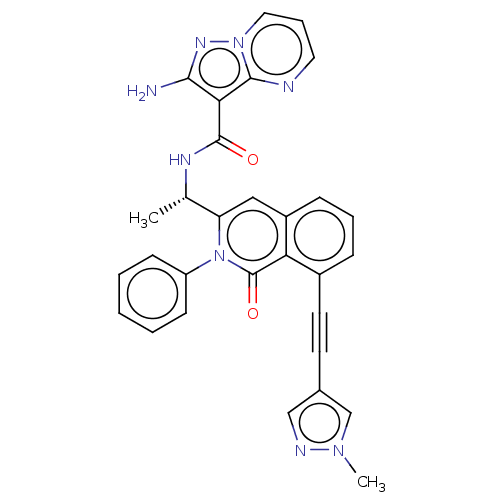
(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells by equilibrium fluorescence titration... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50468033
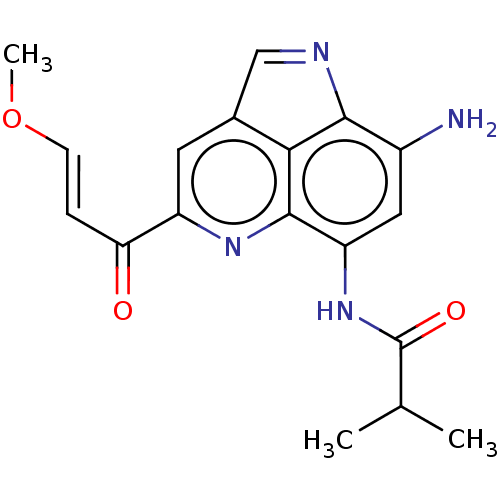
(CHEMBL4293970)Show SMILES CO\C=C\C(=O)c1cc2C=Nc3c(N)cc(NC(=O)C(C)C)c(n1)c23 |c:9| Show InChI InChI=1S/C18H18N4O3/c1-9(2)18(24)22-13-7-11(19)16-15-10(8-20-16)6-12(21-17(13)15)14(23)4-5-25-3/h4-9H,19H2,1-3H3,(H,22,24)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma equilibrate for 6 hrs by scan-kinetic platform assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50468033

(CHEMBL4293970)Show SMILES CO\C=C\C(=O)c1cc2C=Nc3c(N)cc(NC(=O)C(C)C)c(n1)c23 |c:9| Show InChI InChI=1S/C18H18N4O3/c1-9(2)18(24)22-13-7-11(19)16-15-10(8-20-16)6-12(21-17(13)15)14(23)4-5-25-3/h4-9H,19H2,1-3H3,(H,22,24)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma equilibrate for 1 hr by scan-kinetic platform assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50358204
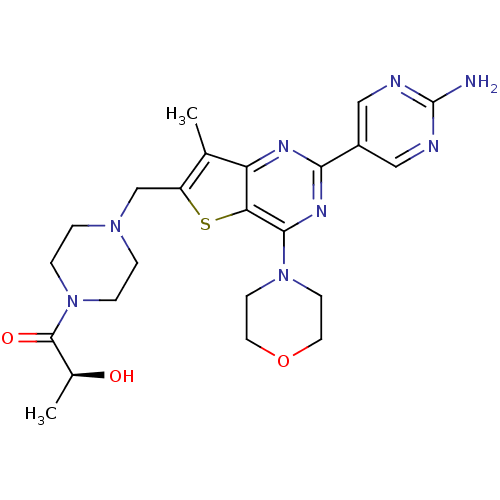
(CHEMBL1922094)Show SMILES C[C@H](O)C(=O)N1CCN(Cc2sc3c(nc(nc3c2C)-c2cnc(N)nc2)N2CCOCC2)CC1 |r| Show InChI InChI=1S/C23H30N8O3S/c1-14-17(13-29-3-5-31(6-4-29)22(33)15(2)32)35-19-18(14)27-20(16-11-25-23(24)26-12-16)28-21(19)30-7-9-34-10-8-30/h11-12,15,32H,3-10,13H2,1-2H3,(H2,24,25,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50468033

(CHEMBL4293970)Show SMILES CO\C=C\C(=O)c1cc2C=Nc3c(N)cc(NC(=O)C(C)C)c(n1)c23 |c:9| Show InChI InChI=1S/C18H18N4O3/c1-9(2)18(24)22-13-7-11(19)16-15-10(8-20-16)6-12(21-17(13)15)14(23)4-5-25-3/h4-9H,19H2,1-3H3,(H,22,24)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma after 1 hr by KINOMEscan assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM315477
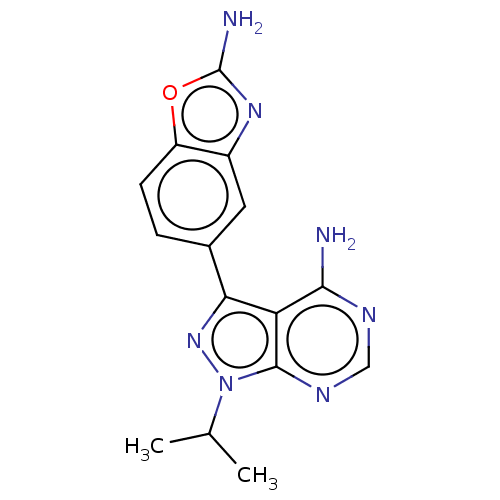
(US10172858, Table 1.1 | US10172858, Table 1.22)Show InChI InChI=1S/C15H15N7O/c1-7(2)22-14-11(13(16)18-6-19-14)12(21-22)8-3-4-10-9(5-8)20-15(17)23-10/h3-7H,1-2H3,(H2,17,20)(H2,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50468033

(CHEMBL4293970)Show SMILES CO\C=C\C(=O)c1cc2C=Nc3c(N)cc(NC(=O)C(C)C)c(n1)c23 |c:9| Show InChI InChI=1S/C18H18N4O3/c1-9(2)18(24)22-13-7-11(19)16-15-10(8-20-16)6-12(21-17(13)15)14(23)4-5-25-3/h4-9H,19H2,1-3H3,(H,22,24)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma equilibrate for 1 hr followed by 30 fold dilution and re-equilibrate for 5 hrs by scan-kinetic platform assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25084
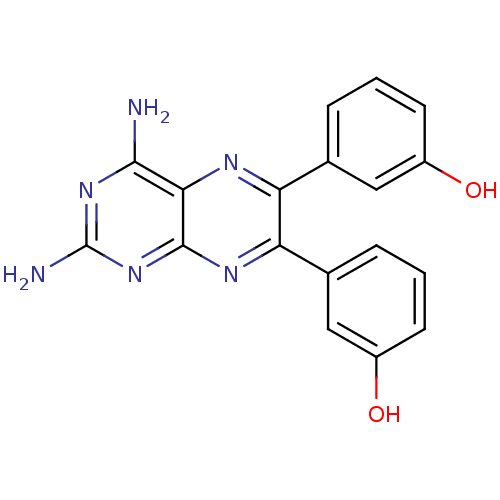
(3-[2,4-diamino-6-(3-hydroxyphenyl)pteridin-7-yl]ph...)Show SMILES Nc1nc(N)c2nc(-c3cccc(O)c3)c(nc2n1)-c1cccc(O)c1 Show InChI InChI=1S/C18H14N6O2/c19-16-15-17(24-18(20)23-16)22-14(10-4-2-6-12(26)8-10)13(21-15)9-3-1-5-11(25)7-9/h1-8,25-26H,(H4,19,20,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CG kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
In vitro renin inhibition was measured at pH 7.4 by using human plasma renin assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50515201

(CHEMBL4453693)Show SMILES C[C@H]1COCCN1c1nc(nc(n1)-c1cnc(N)cc1C(F)F)N1CCOCC1 |r| Show InChI InChI=1S/C18H23F2N7O2/c1-11-10-29-7-4-27(11)18-24-16(13-9-22-14(21)8-12(13)15(19)20)23-17(25-18)26-2-5-28-6-3-26/h8-9,11,15H,2-7,10H2,1H3,(H2,21,22)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human partial length PIK3CG (S144 to A1102 residues) expressed in mammalian expression system by quantitative PCR based... |
J Med Chem 62: 6241-6261 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00525
BindingDB Entry DOI: 10.7270/Q29G5R5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50240973
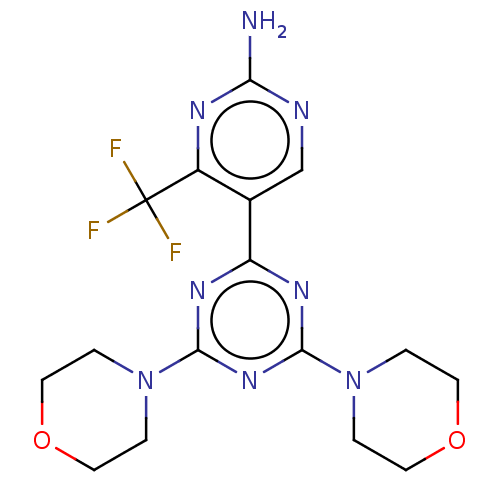
(CHEMBL4102855)Show SMILES Nc1ncc(-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C16H19F3N8O2/c17-16(18,19)11-10(9-21-13(20)22-11)12-23-14(26-1-5-28-6-2-26)25-15(24-12)27-3-7-29-8-4-27/h9H,1-8H2,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM15234
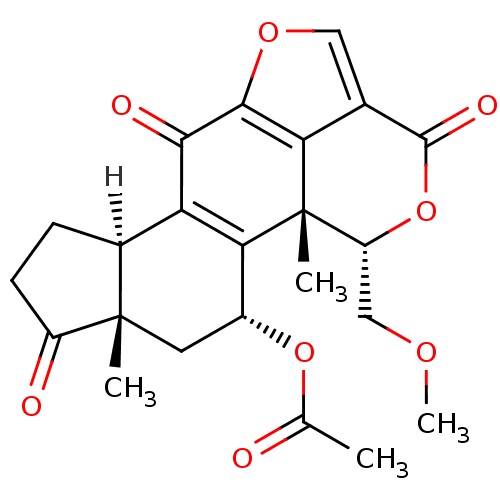
((1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-...)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)C[C@@H](OC(C)=O)C1=C2C(=O)c2occ3c2[C@]1(C)[C@@H](COC)OC3=O |r,c:15| Show InChI InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma after 1 hr by KINOMEscan assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CG kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50308135
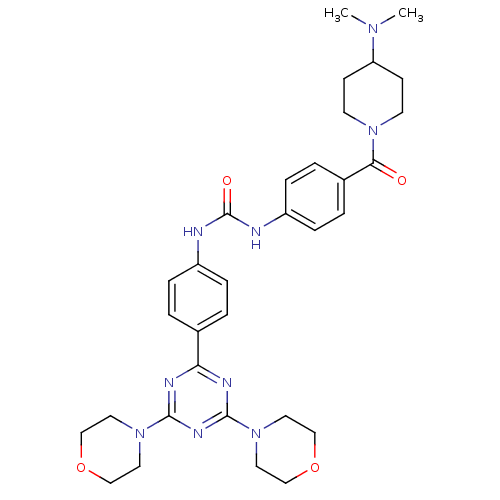
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50240975
(CHEMBL4084907)Show SMILES Nc1cc(c(cn1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C17H20F3N7O2/c18-17(19,20)12-9-13(21)22-10-11(12)14-23-15(26-1-5-28-6-2-26)25-16(24-14)27-3-7-29-8-4-27/h9-10H,1-8H2,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM36409
(2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin...)Show SMILES CC(C)n1nc(-c2cc3cc(O)ccc3[nH]2)c2c(N)ncnc12 Show InChI InChI=1S/C16H16N6O/c1-8(2)22-16-13(15(17)18-7-19-16)14(21-22)12-6-9-5-10(23)3-4-11(9)20-12/h3-8,20,23H,1-2H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM36409

(2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin...)Show SMILES CC(C)n1nc(-c2cc3cc(O)ccc3[nH]2)c2c(N)ncnc12 Show InChI InChI=1S/C16H16N6O/c1-8(2)22-16-13(15(17)18-7-19-16)14(21-22)12-6-9-5-10(23)3-4-11(9)20-12/h3-8,20,23H,1-2H3,(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CG kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CG kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50468033

(CHEMBL4293970)Show SMILES CO\C=C\C(=O)c1cc2C=Nc3c(N)cc(NC(=O)C(C)C)c(n1)c23 |c:9| Show InChI InChI=1S/C18H18N4O3/c1-9(2)18(24)22-13-7-11(19)16-15-10(8-20-16)6-12(21-17(13)15)14(23)4-5-25-3/h4-9H,19H2,1-3H3,(H,22,24)/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a |
University of California San Diego
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma diluted 30 fold and equilibrate for 6 hrs by scan-kinetic platform assay |
J Med Chem 61: 10463-10472 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00975
BindingDB Entry DOI: 10.7270/Q24F1TD1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50555461
(CHEMBL4749655)Show SMILES OCc1cccc(c1)-c1nc(N2CCOCC2)c2ncn(CCCCCCC(=O)NO)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type partial length PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50555464
(CHEMBL4744689)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2ccn(CCCCCCC(=O)NO)c2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human wild type partial length PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.7b01465
BindingDB Entry DOI: 10.7270/Q2765K0S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50380363
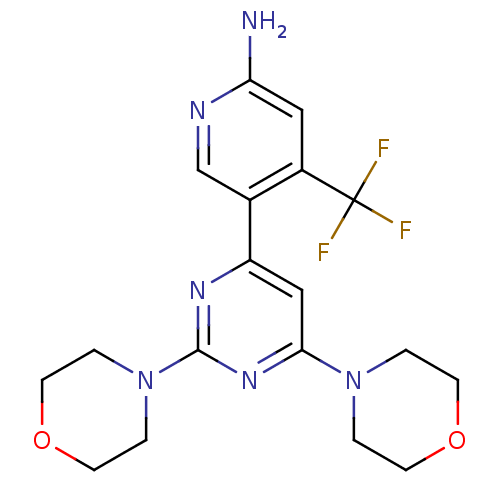
(CHEMBL2017974 | US10173995, Compound 1)Show SMILES Nc1cc(c(cn1)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C18H21F3N6O2/c19-18(20,21)13-9-15(22)23-11-12(13)14-10-16(26-1-5-28-6-2-26)25-17(24-14)27-3-7-29-8-4-27/h9-11H,1-8H2,(H2,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by Kinomescan assay |
J Med Chem 60: 7524-7538 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00930
BindingDB Entry DOI: 10.7270/Q2WM1GKP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.80E+3 | 170 | n/a | n/a | n/a | 7.2 | 20 |
MRC
| Assay Description
Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... |
Mol Cell 6: 909-19 (2000)
Article DOI: 10.1016/s1097-2765(05)00089-4
BindingDB Entry DOI: 10.7270/Q22F7KPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.40E+3 | 210 | n/a | n/a | n/a | 7.2 | 20 |
MRC
| Assay Description
Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... |
Mol Cell 6: 909-19 (2000)
Article DOI: 10.1016/s1097-2765(05)00089-4
BindingDB Entry DOI: 10.7270/Q22F7KPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50271149
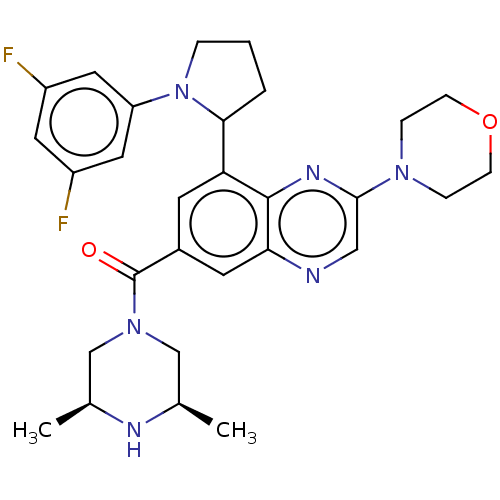
(CHEMBL4125781)Show SMILES C[C@H]1CN(C[C@@H](C)N1)C(=O)c1cc(C2CCCN2c2cc(F)cc(F)c2)c2nc(cnc2c1)N1CCOCC1 |r| Show InChI InChI=1S/C29H34F2N6O2/c1-18-16-36(17-19(2)33-18)29(38)20-10-24(26-4-3-5-37(26)23-13-21(30)12-22(31)14-23)28-25(11-20)32-15-27(34-28)35-6-8-39-9-7-35/h10-15,18-19,26,33H,3-9,16-17H2,1-2H3/t18-,19+,26? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human PIK3Cgamma (S144 to A1102 residues) expressed in mammalian expression system by KINOMEScan assay |
ACS Med Chem Lett 8: 778-780 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00254
BindingDB Entry DOI: 10.7270/Q2TT4TFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288377
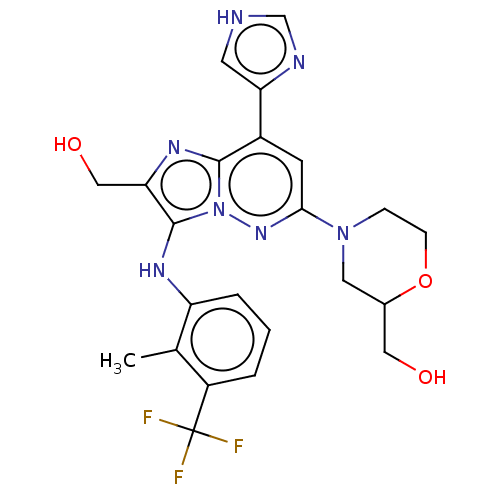
(US10087187, Compound 68)Show SMILES Cc1c(Nc2c(CO)nc3c(cc(nn23)N2CCOC(CO)C2)-c2c[nH]cn2)cccc1C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.80E+3 | 280 | n/a | n/a | n/a | 7.2 | 20 |
MRC
| Assay Description
Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... |
Mol Cell 6: 909-19 (2000)
Article DOI: 10.1016/s1097-2765(05)00089-4
BindingDB Entry DOI: 10.7270/Q22F7KPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.00E+3 | 290 | n/a | n/a | n/a | 7.2 | 20 |
MRC
| Assay Description
Binding was detected as a change in the intrinsic tryptophan fluorescence of the PI3K upon the addition of inhibitor. The inhibitor was incubated wit... |
Mol Cell 6: 909-19 (2000)
Article DOI: 10.1016/s1097-2765(05)00089-4
BindingDB Entry DOI: 10.7270/Q22F7KPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
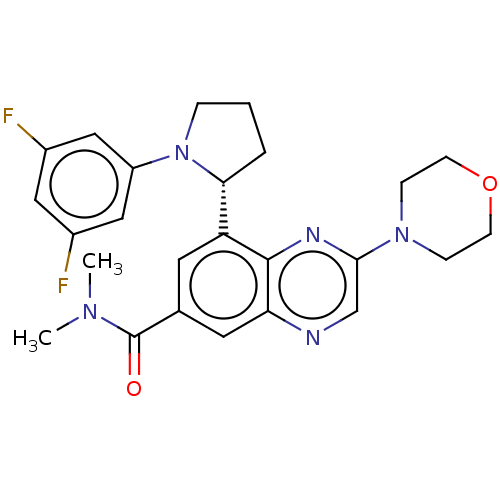
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50271156
(CHEMBL4130282)Show SMILES CN(C)C(=O)c1cc([C@H]2CCCN2c2cc(F)cc(F)c2)c2nc(cnc2c1)N1CCOCC1 |r| Show InChI InChI=1S/C25H27F2N5O2/c1-30(2)25(33)16-10-20(22-4-3-5-32(22)19-13-17(26)12-18(27)14-19)24-21(11-16)28-15-23(29-24)31-6-8-34-9-7-31/h10-15,22H,3-9H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human PIK3Cgamma (S144 to A1102 residues) expressed in mammalian expression system by KINOMEScan assay |
ACS Med Chem Lett 8: 778-780 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00254
BindingDB Entry DOI: 10.7270/Q2TT4TFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
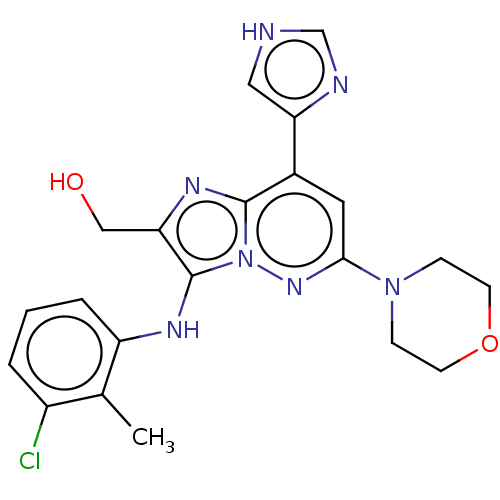
(Homo sapiens (Human)) | BDBM288376
(US10087187, Compound 67)Show SMILES Cc1c(Cl)cccc1Nc1c(CO)nc2c(cc(nn12)N1CCOCC1)-c1c[nH]cn1 Show InChI InChI=1S/C21H22ClN7O2/c1-13-15(22)3-2-4-16(13)25-21-18(11-30)26-20-14(17-10-23-12-24-17)9-19(27-29(20)21)28-5-7-31-8-6-28/h2-4,9-10,12,25,30H,5-8,11H2,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
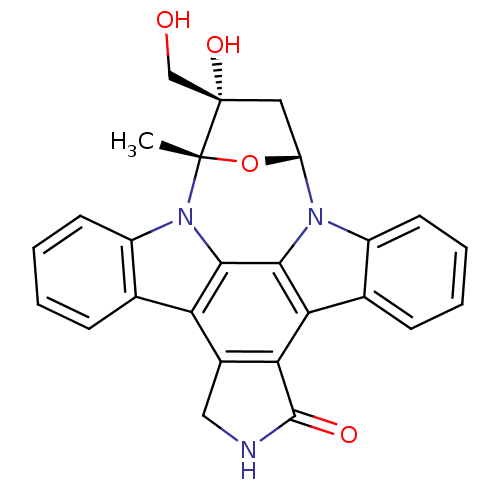
(Homo sapiens (Human)) | BDBM50308060
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for PIK3CG kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to PIK3CG |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
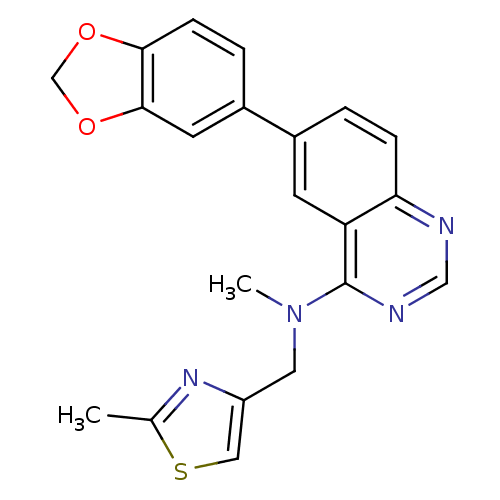
(Homo sapiens (Human)) | BDBM50342916
(6-(benzo[d][1,3]dioxol-5-yl)-N-methyl-N-((2-methyl...)Show SMILES CN(Cc1csc(C)n1)c1ncnc2ccc(cc12)-c1ccc2OCOc2c1 Show InChI InChI=1S/C21H18N4O2S/c1-13-24-16(10-28-13)9-25(2)21-17-7-14(3-5-18(17)22-11-23-21)15-4-6-19-20(8-15)27-12-26-19/h3-8,10-11H,9,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human PIK3CG |
Bioorg Med Chem Lett 21: 3152-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.114
BindingDB Entry DOI: 10.7270/Q2J67H83 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
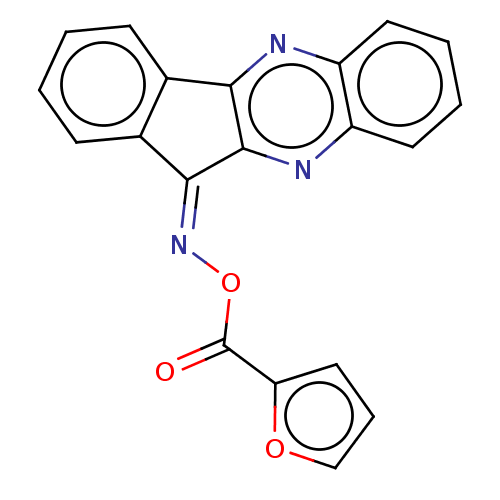
(Homo sapiens (Human)) | BDBM50059657
(CHEMBL3393602)Show SMILES O=C(O\N=C1\c2ccccc2-c2nc3ccccc3nc12)c1ccco1 Show InChI InChI=1S/C20H11N3O3/c24-20(16-10-5-11-25-16)26-23-18-13-7-2-1-6-12(13)17-19(18)22-15-9-4-3-8-14(15)21-17/h1-11H/b23-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls Universit£t T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) by KINOMEScan assay |
J Med Chem 58: 72-95 (2015)
Article DOI: 10.1021/jm501212r
BindingDB Entry DOI: 10.7270/Q2JH3NVZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50059656
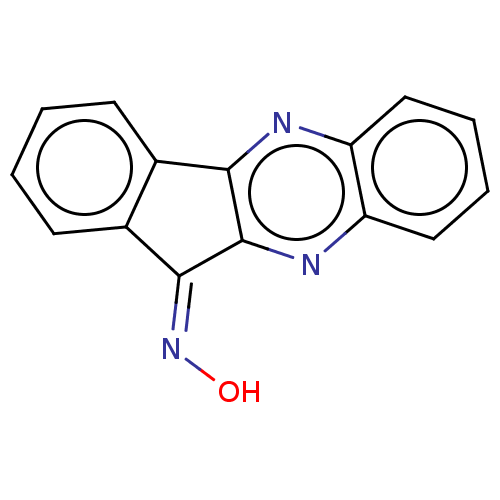
(CHEMBL3393601)Show InChI InChI=1S/C15H9N3O/c19-18-14-10-6-2-1-5-9(10)13-15(14)17-12-8-4-3-7-11(12)16-13/h1-8,19H/b18-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls Universit£t T£bingen
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) by KINOMEScan assay |
J Med Chem 58: 72-95 (2015)
Article DOI: 10.1021/jm501212r
BindingDB Entry DOI: 10.7270/Q2JH3NVZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
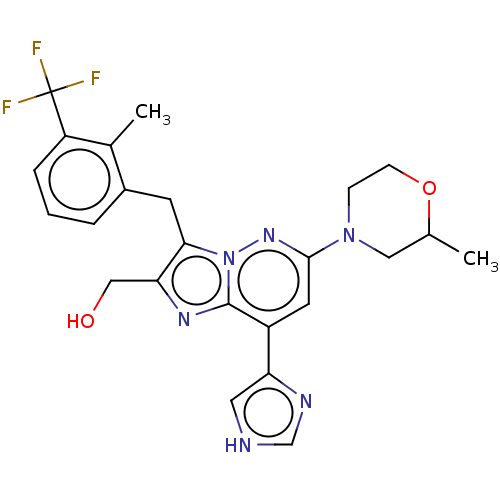
(Homo sapiens (Human)) | BDBM288335
(US10087187, Compound 25 | US10087187, Compound 27)Show SMILES CC1CN(CCO1)c1cc(-c2c[nH]cn2)c2nc(CO)c(Cc3cccc(c3C)C(F)(F)F)n2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50506315
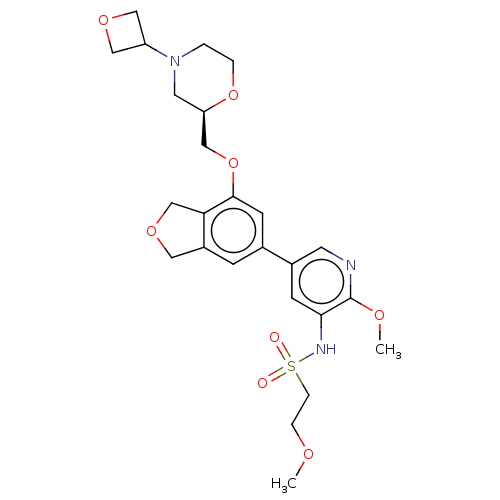
(CHEMBL4579923)Show SMILES COCCS(=O)(=O)Nc1cc(cnc1OC)-c1cc2COCc2c(OC[C@H]2CN(CCO2)C2COC2)c1 |r| Show InChI InChI=1S/C25H33N3O8S/c1-31-5-6-37(29,30)27-23-8-18(10-26-25(23)32-2)17-7-19-12-33-16-22(19)24(9-17)36-15-21-11-28(3-4-35-21)20-13-34-14-20/h7-10,20-21,27H,3-6,11-16H2,1-2H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kgamma in human HL60 cell extract measured after 2 hrs by kinobeads based pull down assay |
J Med Chem 63: 638-655 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01585
BindingDB Entry DOI: 10.7270/Q2F76GVB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50559197
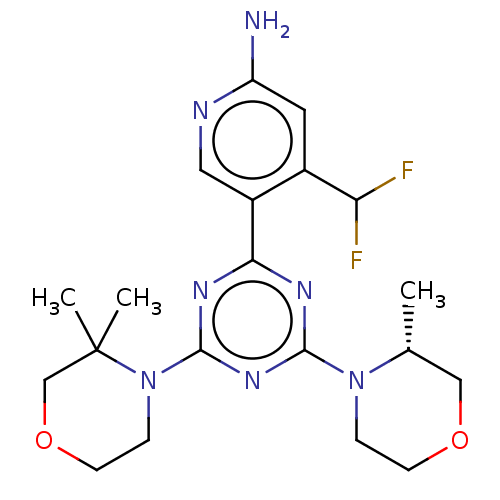
(CHEMBL4741511)Show SMILES C[C@@H]1COCCN1c1nc(nc(n1)-c1cnc(N)cc1C(F)F)N1CCOCC1(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 595 | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to wild-type human partial length PIK3CG (S144 to A1102 residues) expressed in HEK293 cells measured after 1 hr by Kinomescan method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00620
BindingDB Entry DOI: 10.7270/Q2SN0DNS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288331
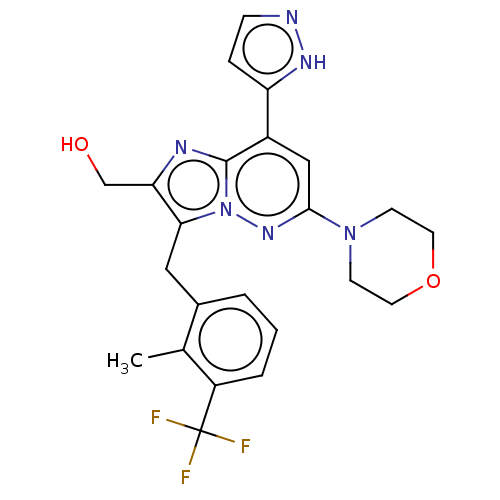
(US10087187, Compound 21)Show SMILES Cc1c(Cc2c(CO)nc3c(cc(nn23)N2CCOCC2)-c2ccn[nH]2)cccc1C(F)(F)F Show InChI InChI=1S/C23H23F3N6O2/c1-14-15(3-2-4-17(14)23(24,25)26)11-20-19(13-33)28-22-16(18-5-6-27-29-18)12-21(30-32(20)22)31-7-9-34-10-8-31/h2-6,12,33H,7-11,13H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50200930
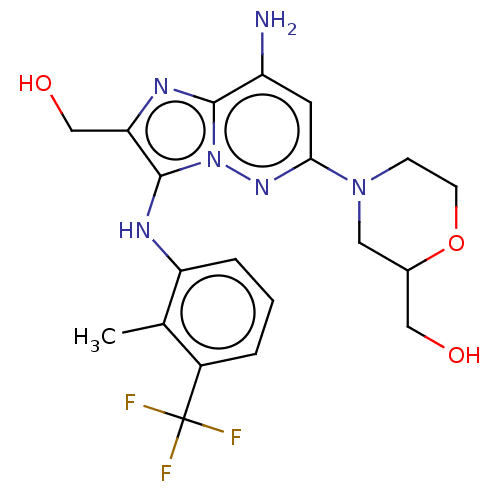
(CHEMBL3928423 | US10087187, Compound 66)Show SMILES Cc1c(Nc2c(CO)nc3c(N)cc(nn23)N2CCOC(CO)C2)cccc1C(F)(F)F Show InChI InChI=1S/C20H23F3N6O3/c1-11-13(20(21,22)23)3-2-4-15(11)25-19-16(10-31)26-18-14(24)7-17(27-29(18)19)28-5-6-32-12(8-28)9-30/h2-4,7,12,25,30-31H,5-6,8-10,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
ACS Med Chem Lett 7: 1012-1013 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00406
BindingDB Entry DOI: 10.7270/Q2N29ZX9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50200930

(CHEMBL3928423 | US10087187, Compound 66)Show SMILES Cc1c(Nc2c(CO)nc3c(N)cc(nn23)N2CCOC(CO)C2)cccc1C(F)(F)F Show InChI InChI=1S/C20H23F3N6O3/c1-11-13(20(21,22)23)3-2-4-15(11)25-19-16(10-31)26-18-14(24)7-17(27-29(18)19)28-5-6-32-12(8-28)9-30/h2-4,7,12,25,30-31H,5-6,8-10,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
ACS Med Chem Lett 7: 1012-1013 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00406
BindingDB Entry DOI: 10.7270/Q2N29ZX9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50200930

(CHEMBL3928423 | US10087187, Compound 66)Show SMILES Cc1c(Nc2c(CO)nc3c(N)cc(nn23)N2CCOC(CO)C2)cccc1C(F)(F)F Show InChI InChI=1S/C20H23F3N6O3/c1-11-13(20(21,22)23)3-2-4-15(11)25-19-16(10-31)26-18-14(24)7-17(27-29(18)19)28-5-6-32-12(8-28)9-30/h2-4,7,12,25,30-31H,5-6,8-10,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288400

(US10087187, Compound 101)Show SMILES CCc1c(F)cccc1Cc1c(CNCCO)nc2c(N)cc(nn12)N1CCOCC1 Show InChI InChI=1S/C22H29FN6O2/c1-2-16-15(4-3-5-17(16)23)12-20-19(14-25-6-9-30)26-22-18(24)13-21(27-29(20)22)28-7-10-31-11-8-28/h3-5,13,25,30H,2,6-12,14,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50200968
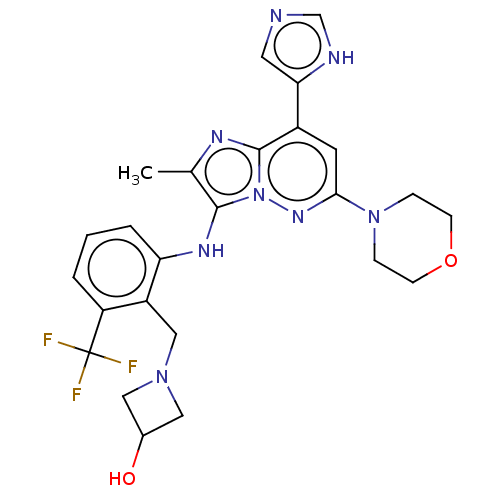
(CHEMBL3983623 | US10087187, Compound 46)Show SMILES Cc1nc2c(cc(nn2c1Nc1cccc(c1CN1CC(O)C1)C(F)(F)F)N1CCOCC1)-c1cnc[nH]1 Show InChI InChI=1S/C25H27F3N8O2/c1-15-23(32-20-4-2-3-19(25(26,27)28)18(20)13-34-11-16(37)12-34)36-24(31-15)17(21-10-29-14-30-21)9-22(33-36)35-5-7-38-8-6-35/h2-4,9-10,14,16,32,37H,5-8,11-13H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC
Curated by ChEMBL
| Assay Description
Binding affinity to human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
ACS Med Chem Lett 7: 1012-1013 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00406
BindingDB Entry DOI: 10.7270/Q2N29ZX9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50200968

(CHEMBL3983623 | US10087187, Compound 46)Show SMILES Cc1nc2c(cc(nn2c1Nc1cccc(c1CN1CC(O)C1)C(F)(F)F)N1CCOCC1)-c1cnc[nH]1 Show InChI InChI=1S/C25H27F3N8O2/c1-15-23(32-20-4-2-3-19(25(26,27)28)18(20)13-34-11-16(37)12-34)36-24(31-15)17(21-10-29-14-30-21)9-22(33-36)35-5-7-38-8-6-35/h2-4,9-10,14,16,32,37H,5-8,11-13H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288347
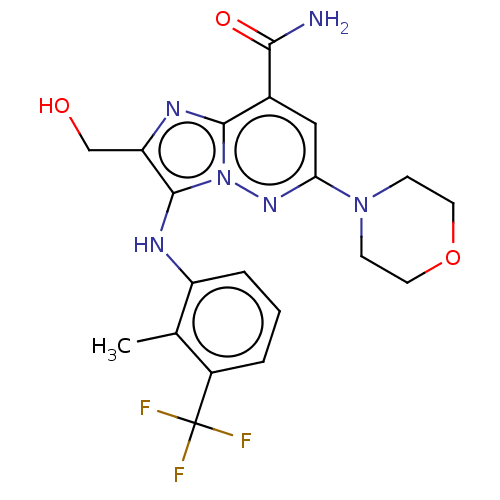
(US10087187, Compound 37)Show SMILES Cc1c(Nc2c(CO)nc3c(cc(nn23)N2CCOCC2)C(N)=O)cccc1C(F)(F)F Show InChI InChI=1S/C20H21F3N6O3/c1-11-13(20(21,22)23)3-2-4-14(11)25-19-15(10-30)26-18-12(17(24)31)9-16(27-29(18)19)28-5-7-32-8-6-28/h2-4,9,25,30H,5-8,10H2,1H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288394
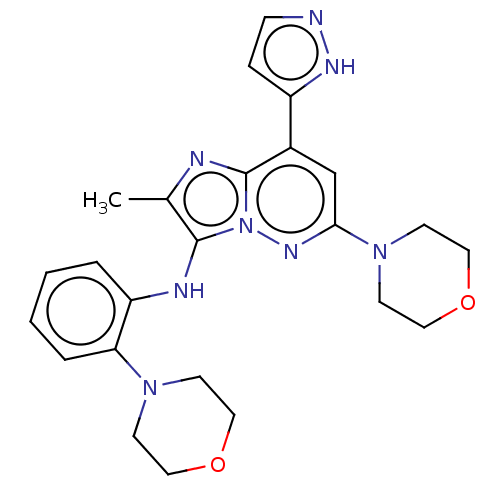
(US10087187, Compound 89)Show SMILES Cc1nc2c(cc(nn2c1Nc1ccccc1N1CCOCC1)N1CCOCC1)-c1ccn[nH]1 Show InChI InChI=1S/C24H28N8O2/c1-17-23(27-20-4-2-3-5-21(20)30-8-12-33-13-9-30)32-24(26-17)18(19-6-7-25-28-19)16-22(29-32)31-10-14-34-15-11-31/h2-7,16,27H,8-15H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288366

(US10087187, Compound 57)Show SMILES CN1CCN(Cc2c(Nc3c(C)nc4c(cc(nn34)N3CCOCC3)-c3c[nH]cn3)cccc2C(F)(F)F)CC1 Show InChI InChI=1S/C27H32F3N9O/c1-18-25(34-22-5-3-4-21(27(28,29)30)20(22)16-37-8-6-36(2)7-9-37)39-26(33-18)19(23-15-31-17-32-23)14-24(35-39)38-10-12-40-13-11-38/h3-5,14-15,17,34H,6-13,16H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM288351
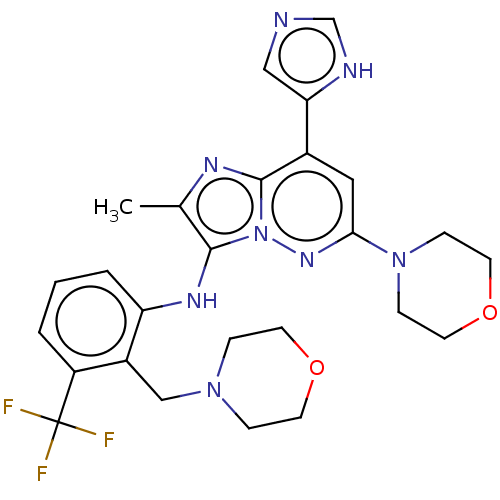
(US10087187, Compound 41)Show SMILES Cc1nc2c(cc(nn2c1Nc1cccc(c1CN1CCOCC1)C(F)(F)F)N1CCOCC1)-c1cnc[nH]1 Show InChI InChI=1S/C26H29F3N8O2/c1-17-24(33-21-4-2-3-20(26(27,28)29)19(21)15-35-5-9-38-10-6-35)37-25(32-17)18(22-14-30-16-31-22)13-23(34-37)36-7-11-39-12-8-36/h2-4,13-14,16,33H,5-12,15H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica NV
US Patent
| Assay Description
Kinase enzyme binding affinities of compounds disclosed herein were determined using the KINOMEscan technology performed by DiscoveRx Corporation, Sa... |
US Patent US10087187 (2018)
BindingDB Entry DOI: 10.7270/Q2M61N9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50271154
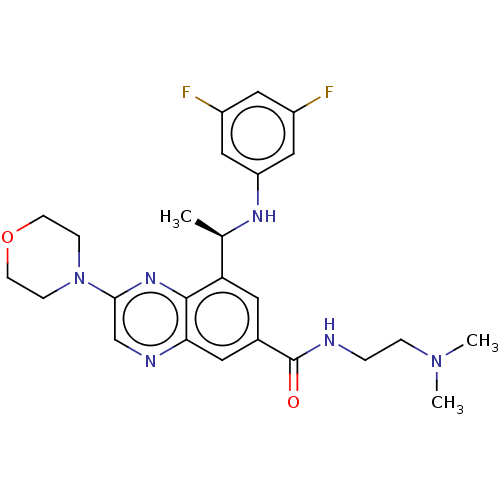
(CHEMBL4128507)Show SMILES C[C@@H](Nc1cc(F)cc(F)c1)c1cc(cc2ncc(nc12)N1CCOCC1)C(=O)NCCN(C)C |r| Show InChI InChI=1S/C25H30F2N6O2/c1-16(30-20-13-18(26)12-19(27)14-20)21-10-17(25(34)28-4-5-32(2)3)11-22-24(21)31-23(15-29-22)33-6-8-35-9-7-33/h10-16,30H,4-9H2,1-3H3,(H,28,34)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 994 | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human PIK3Cgamma (S144 to A1102 residues) expressed in mammalian expression system by KINOMEScan assay |
ACS Med Chem Lett 8: 778-780 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00254
BindingDB Entry DOI: 10.7270/Q2TT4TFM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data