

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573090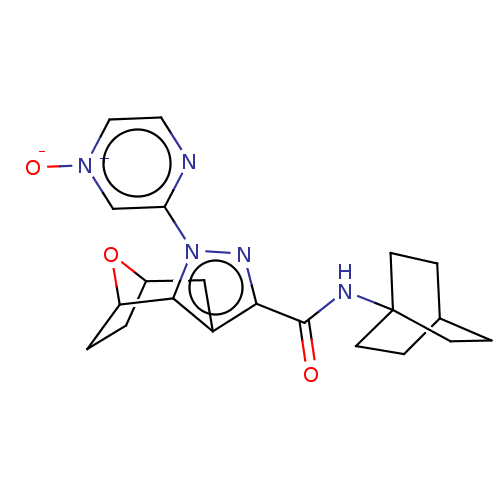 (CHEMBL4847866) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573088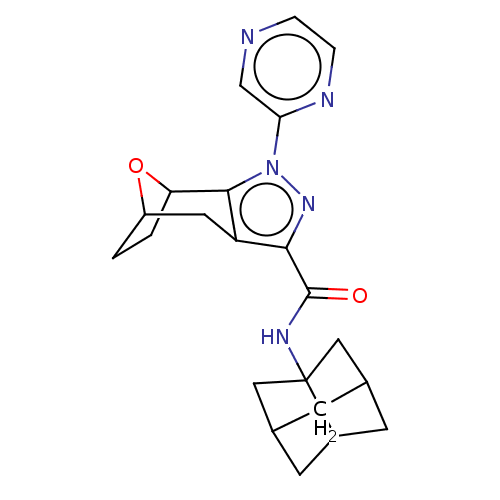 (CHEMBL4873473) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288370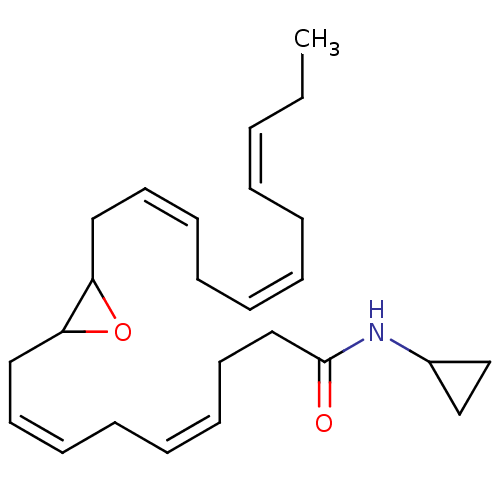 (CHEMBL4169198 | US11472787, Compound 10,11-EDP-CA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288370 (CHEMBL4169198 | US11472787, Compound 10,11-EDP-CA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at N-terminal FLAG-tagged human CB1 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte... | J Med Chem 61: 5569-5579 (2018) Article DOI: 10.1021/acs.jmedchem.8b00243 BindingDB Entry DOI: 10.7270/Q2P84FDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573087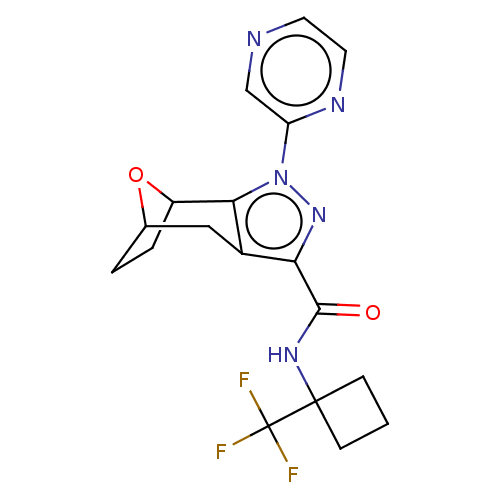 (CHEMBL4862304) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573093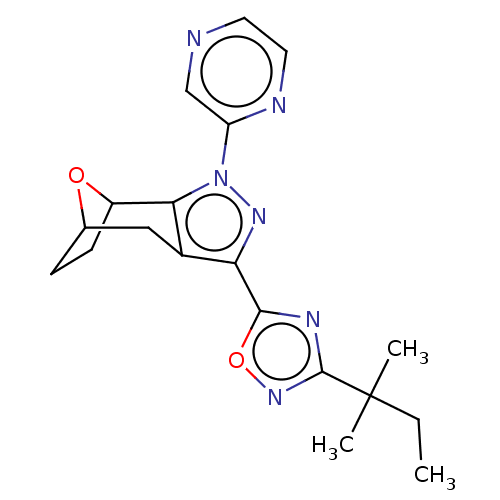 (CHEMBL4875720) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573089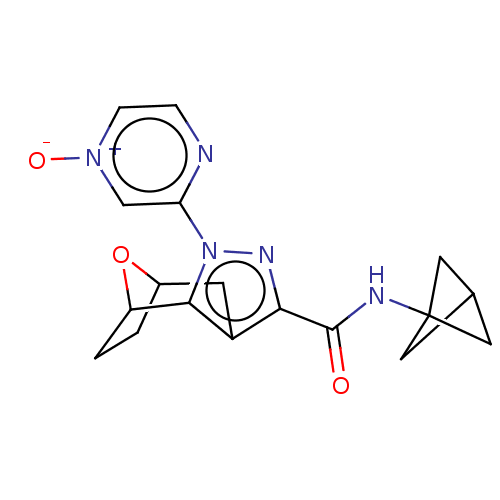 (CHEMBL4846759) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573091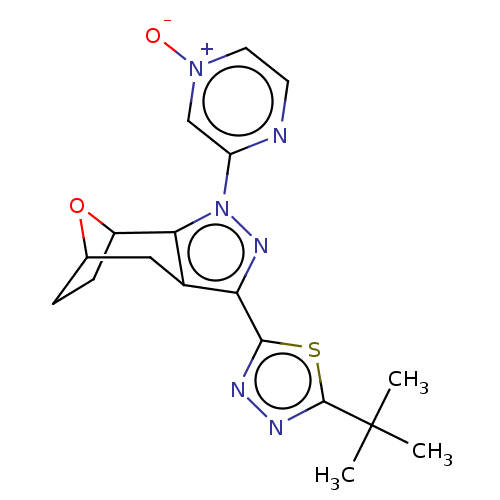 (CHEMBL4865162) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288371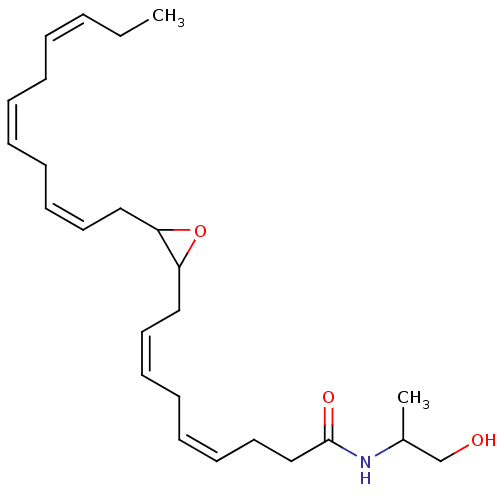 (CHEMBL4177060 | US11472787, Compound 10,11-EDP-IA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at N-terminal FLAG-tagged human CB1 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte... | J Med Chem 61: 5569-5579 (2018) Article DOI: 10.1021/acs.jmedchem.8b00243 BindingDB Entry DOI: 10.7270/Q2P84FDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288371 (CHEMBL4177060 | US11472787, Compound 10,11-EDP-IA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573094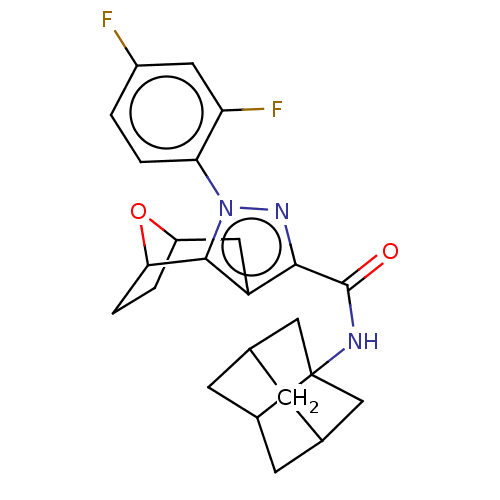 (CHEMBL4872695) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50582406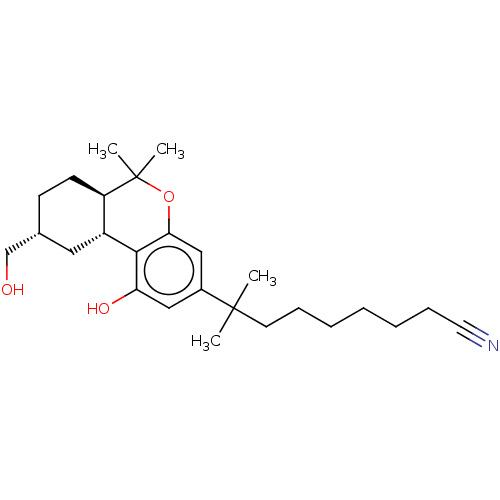 (CHEMBL5091754) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0880 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 3xHA tagged human CB1 receptor expressed in CHO-K1 cells assessed as reduction in forskolin-stimulated cAMP accumulation incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573086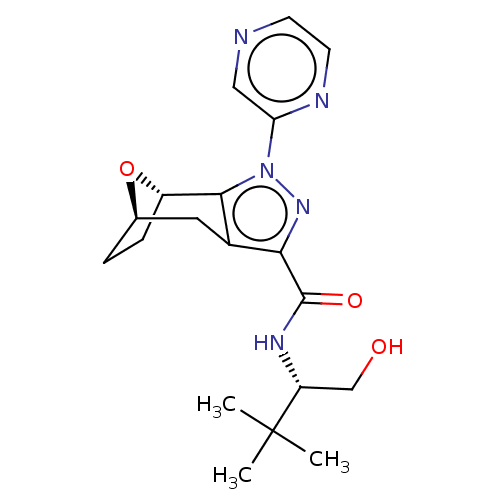 (CHEMBL4871491) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50582401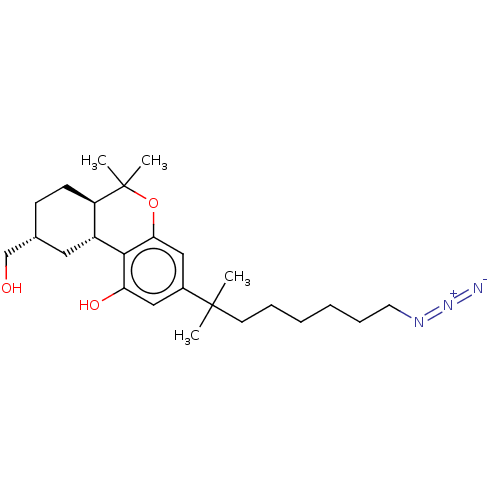 (CHEMBL5081770) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 3xHA tagged human CB1 receptor expressed in CHO-K1 cells assessed as reduction in forskolin-stimulated cAMP accumulation incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inverse agonist activity at human cannabinoid CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation af... | Eur J Med Chem 45: 1133-9 (2010) Article DOI: 10.1016/j.ejmech.2009.12.018 BindingDB Entry DOI: 10.7270/Q2K937P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM532438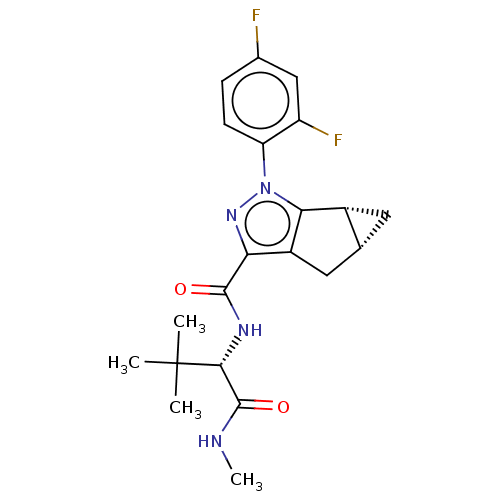 (US11214548, Compound 634) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288369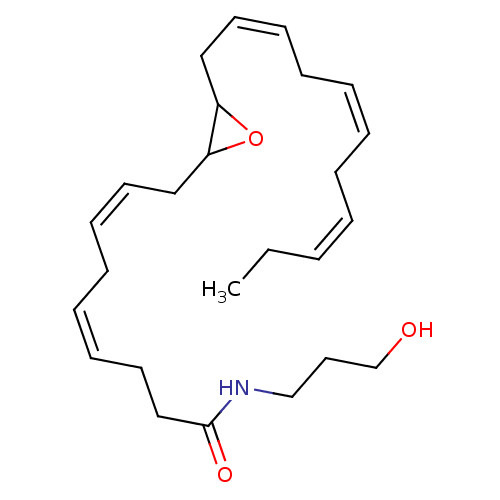 (CHEMBL4172580 | US11472787, Compound 10,11-EDP-NA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288369 (CHEMBL4172580 | US11472787, Compound 10,11-EDP-NA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at N-terminal FLAG-tagged human CB1 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte... | J Med Chem 61: 5569-5579 (2018) Article DOI: 10.1021/acs.jmedchem.8b00243 BindingDB Entry DOI: 10.7270/Q2P84FDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated intracellular cAMP level | J Med Chem 51: 5019-34 (2008) Article DOI: 10.1021/jm800463f BindingDB Entry DOI: 10.7270/Q2W096VS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor expressed in HEK293 cells assessed as reversal of forskolin-evoked cAMP accumulation | J Med Chem 50: 3851-6 (2007) Article DOI: 10.1021/jm070317a BindingDB Entry DOI: 10.7270/Q2K64JWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in HEK293 cells after 24 hrs by luciferase reporter gene assay based Tango beta-arrestin recruitment... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01023 BindingDB Entry DOI: 10.7270/Q21N84WT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267942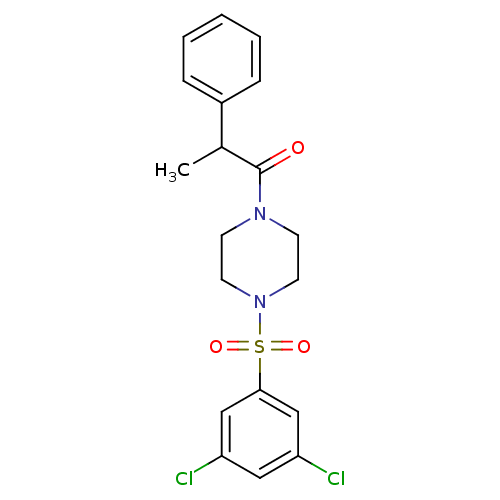 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50041002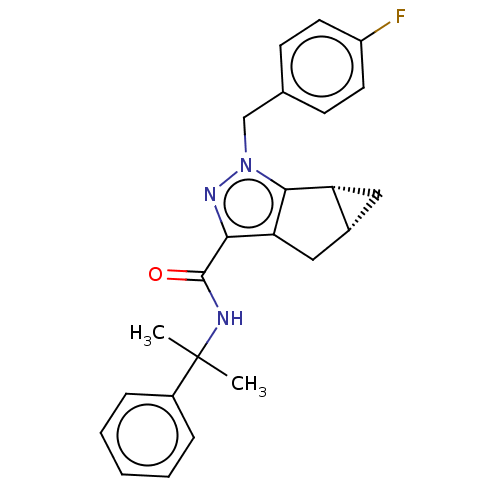 (CHEMBL3354941) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1 receptor expressed in CHOK1 cells assessed as cAMP accumulation by HTRF method | Bioorg Med Chem Lett 25: 322-6 (2014) Article DOI: 10.1016/j.bmcl.2014.11.040 BindingDB Entry DOI: 10.7270/Q21J9CDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50582407 (CHEMBL5071417) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 3xHA tagged human CB1 receptor expressed in CHO-K1 cells assessed as reduction in forskolin-stimulated cAMP accumulation incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50556535 (CHEMBL4782654) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human CB1R expressed in CHO cell membranes preincubated for 30 mins followed by GTPgammaS addition and measured after 90 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112858 BindingDB Entry DOI: 10.7270/Q2SQ943D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288368 (CHEMBL4161934 | US11472787, Compound 10,11-EDP-EA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Agonist activity at N-terminal FLAG-tagged human CB1 receptor transfected in human HTLA cells assessed as induction of beta-arrestin-recruitment afte... | J Med Chem 61: 5569-5579 (2018) Article DOI: 10.1021/acs.jmedchem.8b00243 BindingDB Entry DOI: 10.7270/Q2P84FDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50582405 (CHEMBL5074603) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 3xHA tagged human CB1 receptor expressed in CHO-K1 cells assessed as reduction in forskolin-stimulated cAMP accumulation incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50607229 (CHEMBL5219235) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00969 BindingDB Entry DOI: 10.7270/Q2K35ZSQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50288368 (CHEMBL4161934 | US11472787, Compound 10,11-EDP-EA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding of the parent compounds to putative receptors CNR1 and CNR2 (cannabinoid receptors 1& 2) was measured by a Presto-Tango assay (FIG. 5A and FI... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Activity at hemagglutinin-tagged human CB1 receptor expressed in HEK293 cells assessed as effect on forskolin-induced cAMP accumulation in presence o... | J Med Chem 58: 5979-88 (2015) Article DOI: 10.1021/acs.jmedchem.5b00579 BindingDB Entry DOI: 10.7270/Q2PG1TG7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267257 (4-(2-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267543 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM143560 (US9682955, 58) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | 27 |
Janssen Pharmaceutica NV US Patent | Assay Description The following mixtures and buffer solutions were prepared: (a) Buffer 1: HBSS (Mediatech Cat#21-023-CV) with 5 mM HEPES (1 mM stock, Gibco BRL Cat#15... | US Patent US9682955 (2017) BindingDB Entry DOI: 10.7270/Q2707ZM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267773 (CHEMBL489429 | cyclohexyl(4-(3,5-dichlorophenylsul...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50063927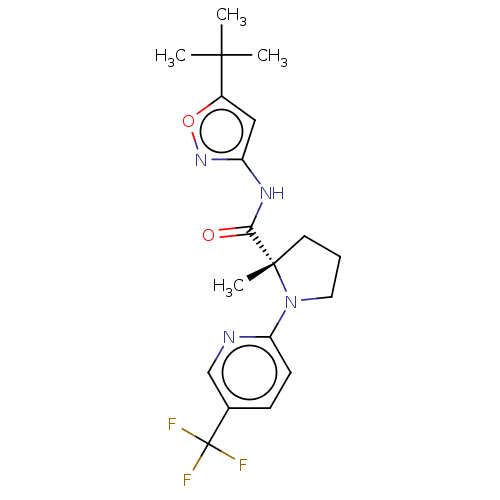 (CHEMBL3400946) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation after 30 mins | Bioorg Med Chem Lett 25: 575-80 (2015) Article DOI: 10.1016/j.bmcl.2014.12.033 BindingDB Entry DOI: 10.7270/Q2222WGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267258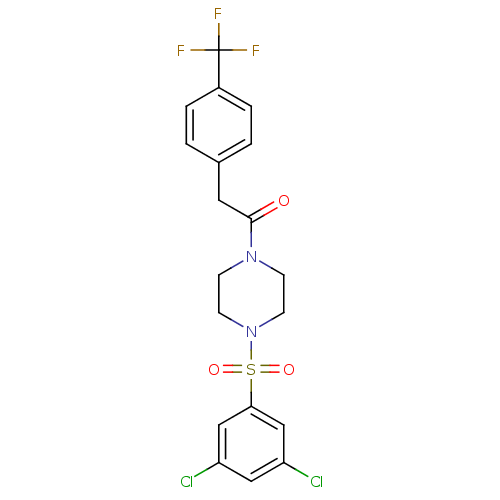 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50582404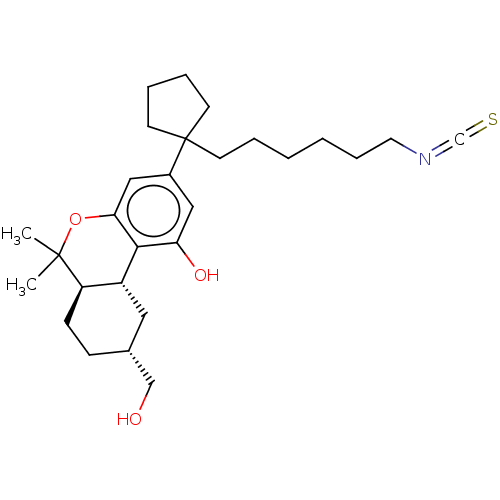 (CHEMBL5092703) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at 3xHA tagged human CB1 receptor expressed in CHO-K1 cells assessed as reduction in forskolin-stimulated cAMP accumulation incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50573085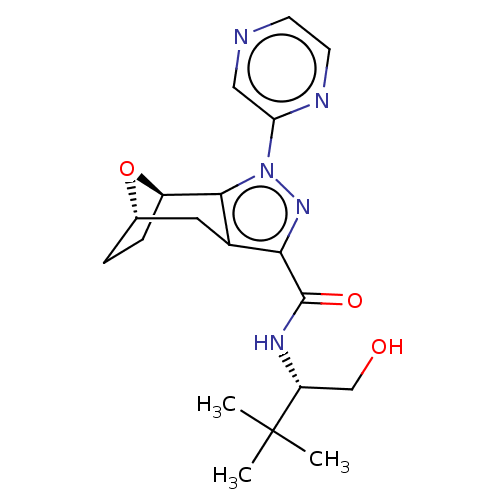 (CHEMBL4871528) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as increase in cAMP level incubated for 20 mins measured after 60 mins addi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00331 BindingDB Entry DOI: 10.7270/Q2JH3R00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067498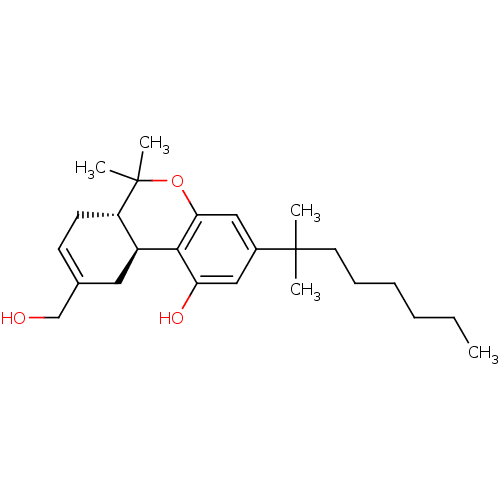 ((6aS,10aS)-3-(1,1-Dimethyl-heptyl)-9-hydroxymethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Activity at human CB1 receptor by [35S]GTP-gamma-S binding stimulation assay | J Med Chem 48: 7486-90 (2005) Article DOI: 10.1021/jm0503906 BindingDB Entry DOI: 10.7270/Q22N5338 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267774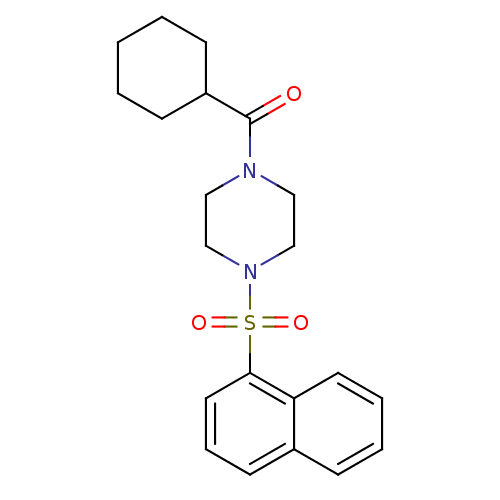 (CHEMBL523100 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Universit£ catholique de Louvain Curated by ChEMBL | Assay Description Potency at human CB1 receptor in a [35S]GTP-gamma-S functional assay | J Med Chem 49: 872-82 (2006) Article DOI: 10.1021/jm050484f BindingDB Entry DOI: 10.7270/Q28916NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267903 (CHEMBL525204 | cyclohexyl(2,2-dimethyl-4-(naphthal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM532441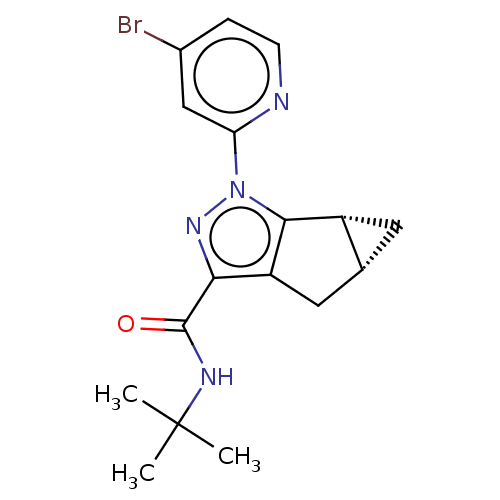 (US11214548, Compound 673) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.644 | n/a | n/a | n/a | n/a |
TBA | Assay Description Table D: Radioligand binding assays for human CB2 receptors were performed using two different agonist radioligands, [3H]CP55,940 and [3H]WIN55,212-2... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BR8WC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590329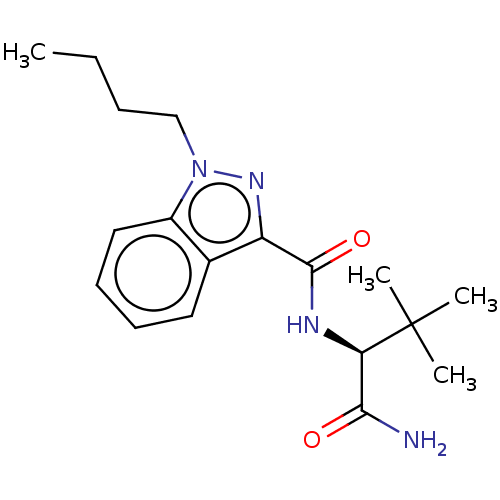 (CHEMBL5169682) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50590329 (CHEMBL5169682) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00242b BindingDB Entry DOI: 10.7270/Q2G73JQJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29076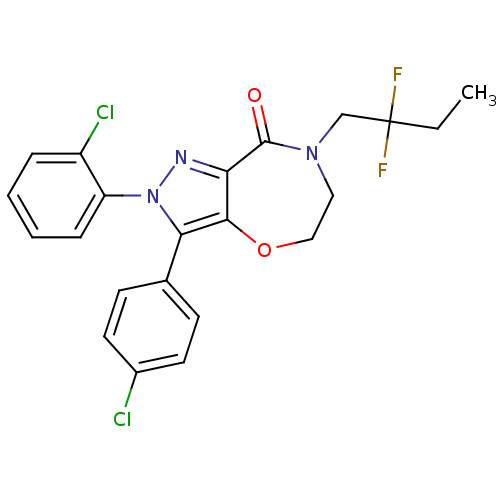 (ether-based lactam, 19e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -12.3 | n/a | n/a | 0.700 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Agonist activity at human CB1 receptor transfected in CHO cell membranes by [35S]GTPgammaS binding assay | Eur J Med Chem 58: 30-43 (2012) Article DOI: 10.1016/j.ejmech.2012.09.035 BindingDB Entry DOI: 10.7270/Q2JD4XW2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29070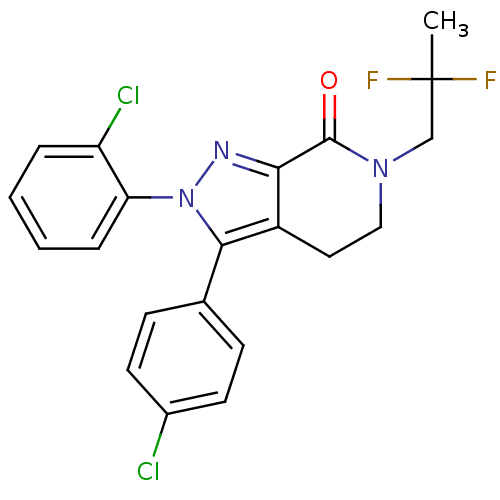 (lactam-based compound, 12i) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -12.7 | n/a | n/a | 0.800 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075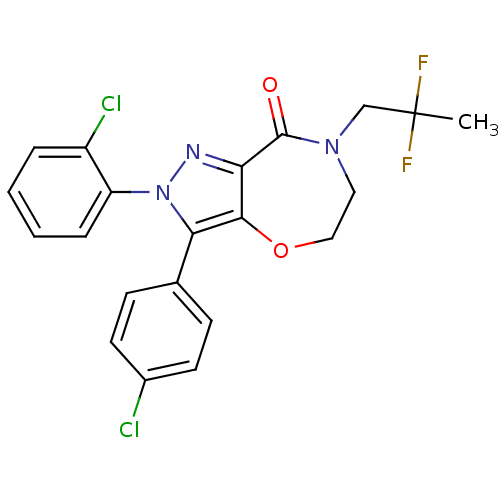 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -12.5 | n/a | n/a | 0.820 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50312599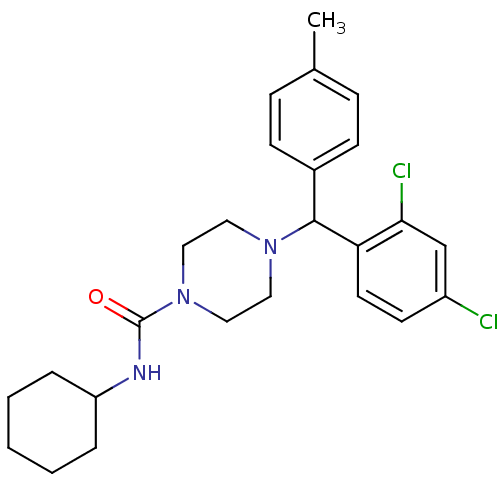 (CHEMBL1093735 | N-Cyclohexyl-4-[(2,4-dichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inverse agonist activity at human cannabinoid CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation af... | Eur J Med Chem 45: 1133-9 (2010) Article DOI: 10.1016/j.ejmech.2009.12.018 BindingDB Entry DOI: 10.7270/Q2K937P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4421 total ) | Next | Last >> |