 Found 4431 hits of ic50 for UniProtKB: P18031
Found 4431 hits of ic50 for UniProtKB: P18031 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition against purified catalytic subunit of protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition against purified catalytic subunit of protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protein phosphatase 1 was determined; 0.5-1.0 |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50366883
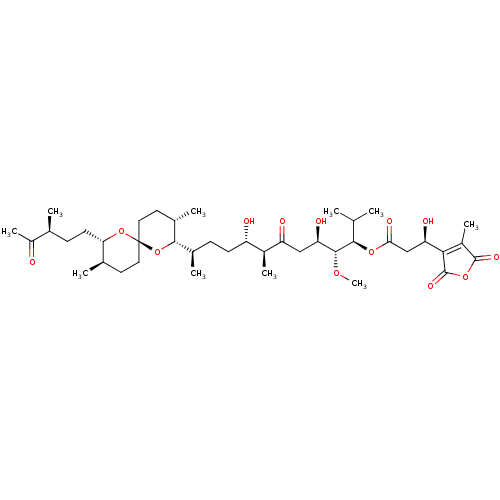
(TAUTOMYCIN)Show SMILES CO[C@H]([C@H](O)CC(=O)[C@@H](C)[C@@H](O)CC[C@@H](C)[C@@H]1O[C@]2(CC[C@@H](C)[C@H](CC[C@H](C)C(C)=O)O2)CC[C@@H]1C)[C@H](OC(=O)C[C@@H](O)C1=C(C)C(=O)OC1=O)C(C)C |r,c:45| Show InChI InChI=1S/C41H66O13/c1-21(2)36(51-34(47)20-31(45)35-27(8)39(48)52-40(35)49)38(50-10)32(46)19-30(44)26(7)29(43)13-11-24(5)37-25(6)16-18-41(54-37)17-15-23(4)33(53-41)14-12-22(3)28(9)42/h21-26,29,31-33,36-38,43,45-46H,11-20H2,1-10H3/t22-,23+,24+,25-,26-,29-,31+,32+,33-,36+,37-,38+,41+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50212842

(Motuporin)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@@H](NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O)C(C)C Show InChI InChI=1S/C40H57N5O10/c1-10-30-37(49)44-34(40(53)54)26(7)36(48)43-33(22(2)3)38(50)41-28(25(6)35(47)42-29(39(51)52)18-19-32(46)45(30)8)17-16-23(4)20-24(5)31(55-9)21-27-14-12-11-13-15-27/h10-17,20,22,24-26,28-29,31,33-34H,18-19,21H2,1-9H3,(H,41,50)(H,42,47)(H,43,48)(H,44,49)(H,51,52)(H,53,54)/b17-16+,23-20+,30-10-/t24-,25-,26-,28-,29+,31-,33-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition against purified catalytic subunit of protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50579994
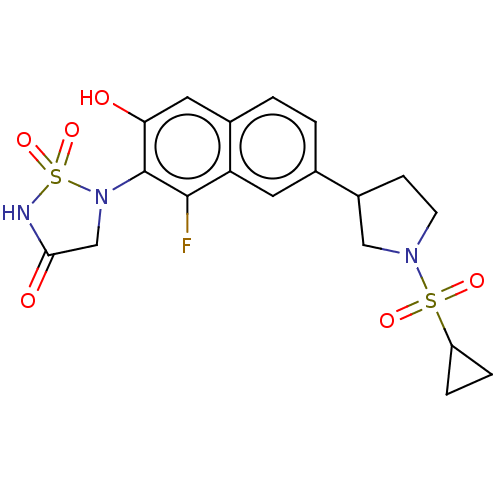
(CHEMBL5070507)Show SMILES Oc1cc2ccc(cc2c(F)c1N1CC(=O)NS1(=O)=O)C1CCN(C1)S(=O)(=O)C1CC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50580005
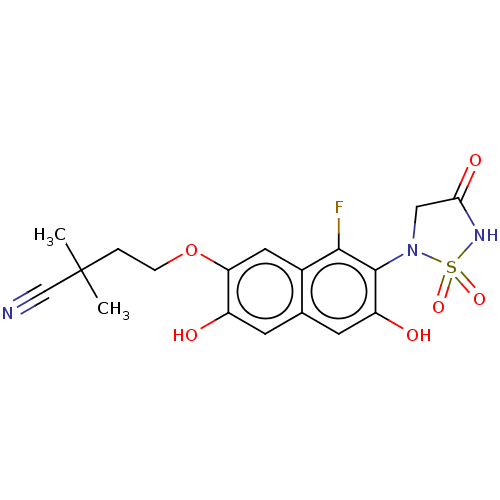
(CHEMBL5093253)Show SMILES CC(C)(CCOc1cc2c(F)c(N3CC(=O)NS3(=O)=O)c(O)cc2cc1O)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50580003
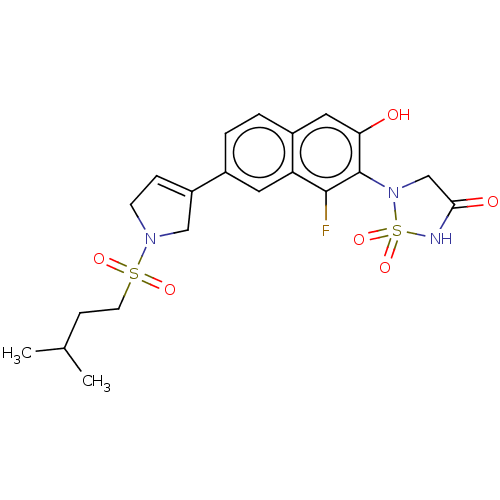
(CHEMBL5080612)Show SMILES CC(C)CCS(=O)(=O)N1CC=C(C1)c1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |c:10| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50580002

(CHEMBL5084380)Show SMILES CCS(=O)(=O)N1CC=C(C1)c1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |c:7| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50580000
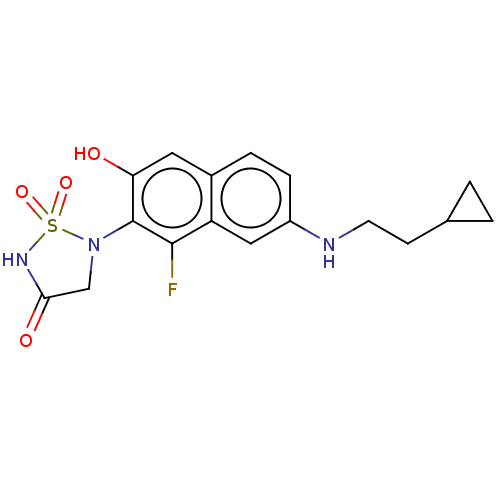
(CHEMBL5076419)Show SMILES Oc1cc2ccc(NCCC3CC3)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50579999
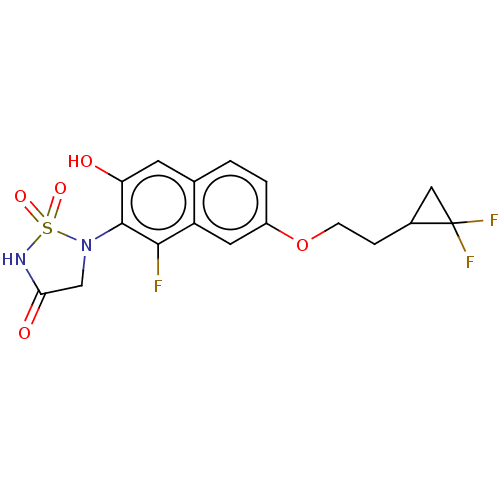
(CHEMBL5075269)Show SMILES Oc1cc2ccc(OCCC3CC3(F)F)cc2c(F)c1N1CC(=O)NS1(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50579995

(CHEMBL5085502)Show SMILES CC(C)(CCOc1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1)C#N | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50579996

(CHEMBL5092308)Show SMILES Oc1cc2ccc(cc2c(F)c1N1CC(=O)NS1(=O)=O)-c1cnn(CC2CC2)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50579997

(CHEMBL5085615)Show SMILES C[C@H](O)CCOc1ccc2cc(O)c(N3CC(=O)NS3(=O)=O)c(F)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTPN1 (unknown origin) by Mobility shift assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00678
BindingDB Entry DOI: 10.7270/Q2SB49MH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity against protein phosphatase 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061067

(15-(3-Guanidino-propyl)-8-isobutyl-18-((1E,3E)-6-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C49H74N10O12/c1-26(2)23-37-46(66)58-40(48(69)70)30(6)42(62)55-35(17-14-22-52-49(50)51)45(65)54-34(19-18-27(3)24-28(4)38(71-10)25-33-15-12-11-13-16-33)29(5)41(61)56-36(47(67)68)20-21-39(60)59(9)32(8)44(64)53-31(7)43(63)57-37/h11-13,15-16,18-19,24,26,28-31,34-38,40H,8,14,17,20-23,25H2,1-7,9-10H3,(H,53,64)(H,54,65)(H,55,62)(H,56,61)(H,57,63)(H,58,66)(H,67,68)(H,69,70)(H4,50,51,52)/b19-18+,27-24+/t28-,29-,30-,31+,34-,35-,36+,37-,38-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50611797
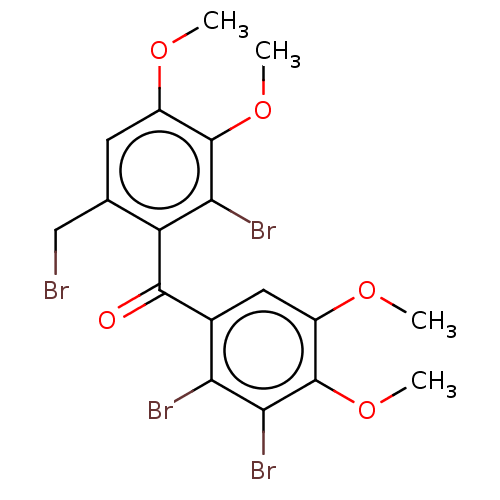
(CHEMBL5270520) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061069
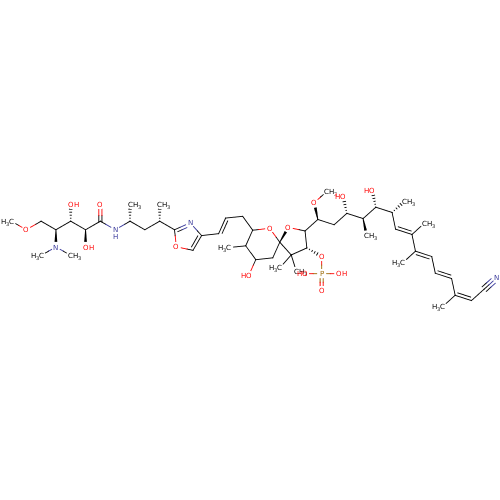
(CHEMBL384277 | Calyculin C)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)N[C@H](C)C[C@H](C)c1nc(\C=C\CC2O[C@]3(CC(O)C2C)OC([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C Show InChI InChI=1S/C51H83N4O15P/c1-29(21-22-52)17-15-18-30(2)31(3)23-32(4)43(58)36(8)39(56)25-42(66-14)46-47(70-71(62,63)64)50(9,10)51(69-46)26-40(57)35(7)41(68-51)20-16-19-37-27-67-49(54-37)33(5)24-34(6)53-48(61)45(60)44(59)38(28-65-13)55(11)12/h15-19,21,23,27,32-36,38-47,56-60H,20,24-26,28H2,1-14H3,(H,53,61)(H2,62,63,64)/b17-15+,19-16+,29-21-,30-18+,31-23+/t32-,33+,34-,35?,36+,38+,39+,40?,41?,42+,43-,44+,45+,46?,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50202437
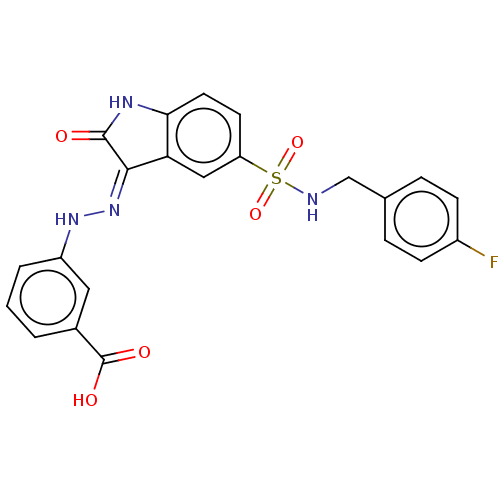
(CHEMBL502013)Show SMILES OC(=O)c1cccc(N\N=C2/C(=O)Nc3ccc(cc23)S(=O)(=O)NCc2ccc(F)cc2)c1 Show InChI InChI=1S/C22H17FN4O5S/c23-15-6-4-13(5-7-15)12-24-33(31,32)17-8-9-19-18(11-17)20(21(28)25-19)27-26-16-3-1-2-14(10-16)22(29)30/h1-11,24,26H,12H2,(H,29,30)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50069691
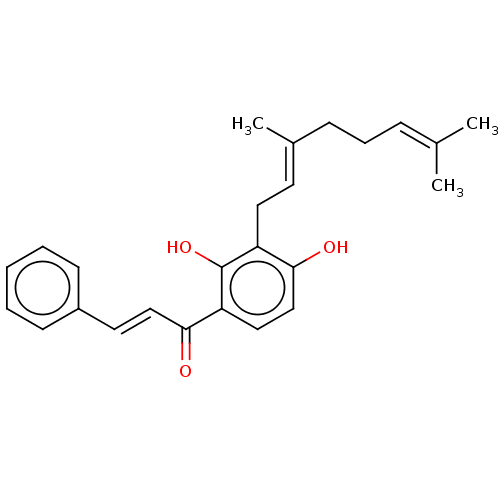
(Xanthoangelol | Xanthoangerol)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccccc2)c1-[#8] Show InChI InChI=1S/C25H28O3/c1-18(2)8-7-9-19(3)12-14-21-24(27)17-15-22(25(21)28)23(26)16-13-20-10-5-4-6-11-20/h4-6,8,10-13,15-17,27-28H,7,9,14H2,1-3H3/b16-13+,19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50610516
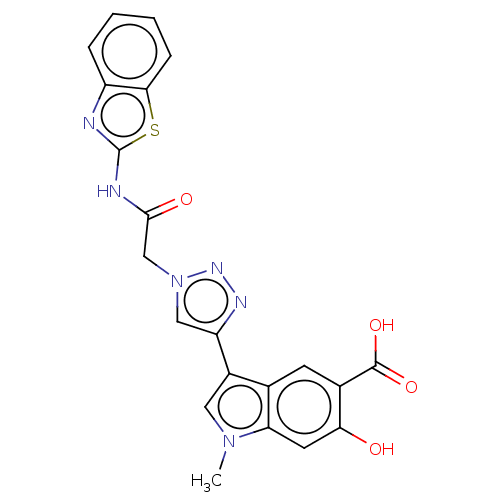
(CHEMBL5287377) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
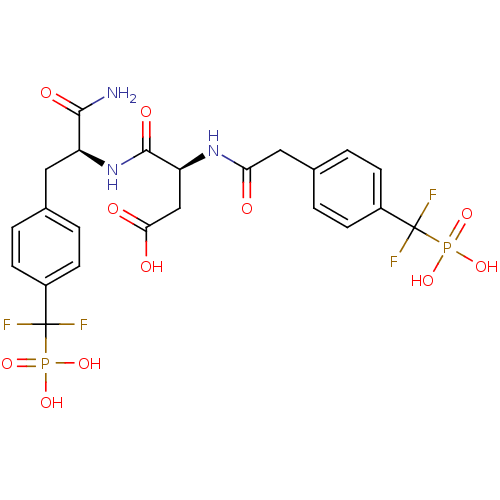
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599
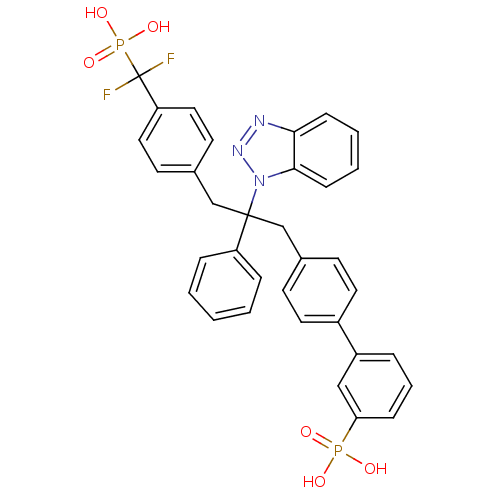
(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged PTP1B (1 to 321 residues) (unknown origin) expressed in bacterial expression system by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50061066

((5R,6S,9S,12S,13S,16R)-2-Eth-(Z)-ylidene-9-(3-guan...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](C)[C@@H](NC(=O)\C(=C\C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C41H60N8O10/c1-8-31-38(54)48-34(40(57)58)26(5)36(52)46-29(15-12-20-44-41(42)43)37(53)45-28(25(4)35(51)47-30(39(55)56)18-19-33(50)49(31)6)17-16-23(2)21-24(3)32(59-7)22-27-13-10-9-11-14-27/h8-11,13-14,16-17,21,24-26,28-30,32,34H,12,15,18-20,22H2,1-7H3,(H,45,53)(H,46,52)(H,47,51)(H,48,54)(H,55,56)(H,57,58)(H4,42,43,44)/b17-16+,23-21+,31-8-/t24-,25-,26-,28-,29-,30+,32-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition activity against protein phosphatase 1 (PP1), activity taken from literature |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13599

(3-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES OP(O)(=O)c1cccc(c1)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H29F2N3O6P2/c35-34(36,47(43,44)45)29-19-15-25(16-20-29)23-33(28-8-2-1-3-9-28,39-32-12-5-4-11-31(32)37-38-39)22-24-13-17-26(18-14-24)27-7-6-10-30(21-27)46(40,41)42/h1-21H,22-23H2,(H2,40,41,42)(H2,43,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124215
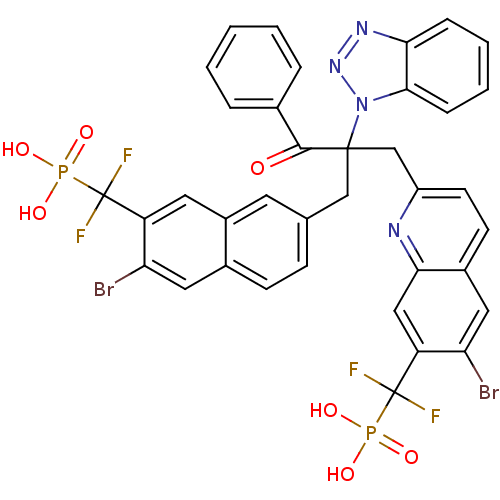
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C37H26Br2F4N4O7P2/c38-29-16-23-11-10-21(14-25(23)15-27(29)36(40,41)55(49,50)51)19-35(34(48)22-6-2-1-3-7-22,47-33-9-5-4-8-31(33)45-46-47)20-26-13-12-24-17-30(39)28(18-32(24)44-26)37(42,43)56(52,53)54/h1-18H,19-20H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50110676

(CHEMBL280487 | okadaic acid)Show SMILES C[C@@H](C[C@H](O)[C@H]1O[C@@H]2CC[C@@]3(CC[C@@H](O3)\C=C\[C@@H](C)[C@@H]3CC(C)=C[C@@]4(O[C@H](C[C@@](C)(O)C(O)=O)CC[C@H]4O)O3)O[C@H]2[C@H](O)C1=C)[C@H]1O[C@@]2(CCCCO2)CC[C@H]1C |r,c:23| Show InChI InChI=1S/C44H68O13/c1-25-21-34(55-44(23-25)35(46)12-11-31(54-44)24-41(6,50)40(48)49)26(2)9-10-30-14-18-43(53-30)19-15-33-39(57-43)36(47)29(5)38(52-33)32(45)22-28(4)37-27(3)13-17-42(56-37)16-7-8-20-51-42/h9-10,23,26-28,30-39,45-47,50H,5,7-8,11-22,24H2,1-4,6H3,(H,48,49)/b10-9+/t26-,27-,28+,30+,31+,32+,33-,34+,35-,36-,37+,38+,39-,41-,42+,43-,44-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition against purified catalytic subunit of protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124216
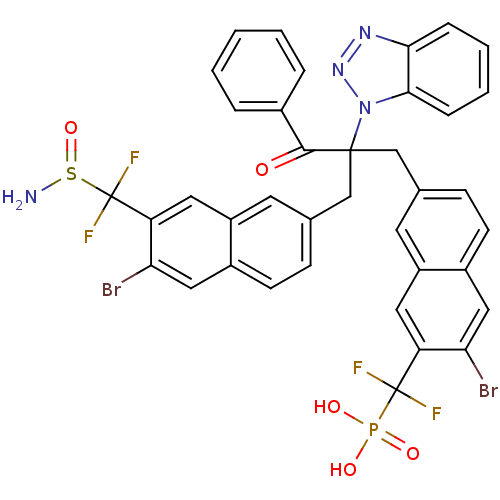
(({7-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N4O5PS/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)54(50,51)52)20-36(35(49)24-6-2-1-3-7-24,48-34-9-5-4-8-33(34)46-47-48)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)55(45)53/h1-19H,20-21,45H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124221
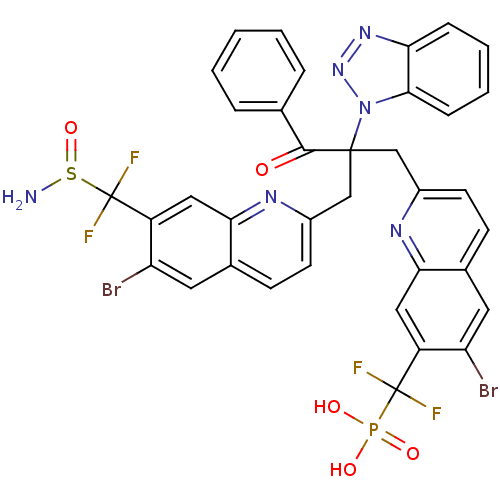
(({2-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C36H25Br2F4N6O5PS/c37-27-14-21-10-12-23(44-30(21)16-25(27)35(39,40)54(50,51)52)18-34(33(49)20-6-2-1-3-7-20,48-32-9-5-4-8-29(32)46-47-48)19-24-13-11-22-15-28(38)26(17-31(22)45-24)36(41,42)55(43)53/h1-17H,18-19,43H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50104694
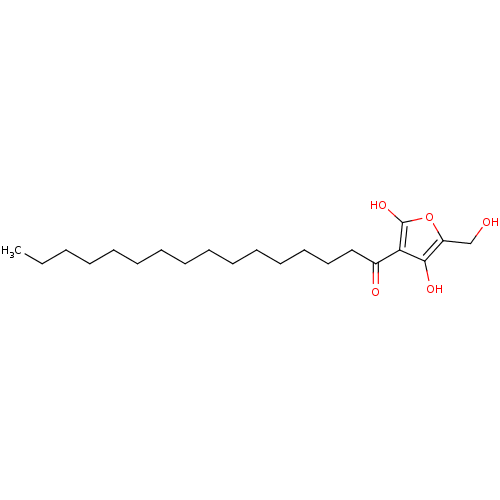
((R)-4-hydroxy-5-(hydroxymethyl)-3-palmitoylfuran-2...)Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h22,24-25H,2-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B |
Bioorg Med Chem Lett 16: 3061-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.053
BindingDB Entry DOI: 10.7270/Q29024KF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50612109

(CHEMBL5275241) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Xi'an Jiaotong University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
Eur J Med Chem 122: 756-769 (2016)
Article DOI: 10.1016/j.ejmech.2016.05.060
BindingDB Entry DOI: 10.7270/Q25Q4Z2D |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50535627
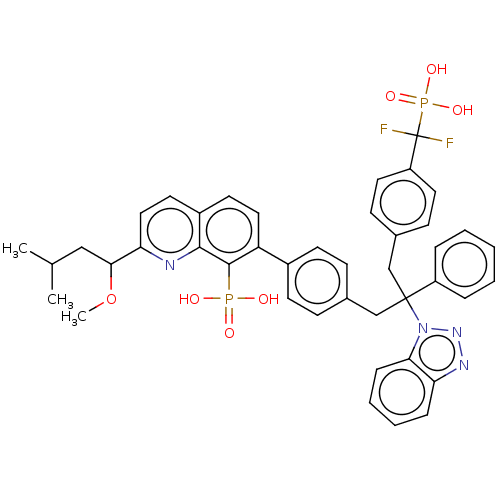
(CHEMBL4542052)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(CC(Cc4ccc(cc4)C(F)(F)P(O)(O)=O)(c4ccccc4)n4nnc5ccccc45)cc3)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)25-39(56-3)37-24-20-32-19-23-35(41(40(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(33-9-5-4-6-10-33,49-38-12-8-7-11-36(38)47-48-49)27-30-15-21-34(22-16-30)43(44,45)58(53,54)55/h4-24,28,39H,25-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Sci-Tech University
Curated by ChEMBL
| Assay Description
Inhibition of human FLAG-tagged PTP1B catalytic domain (1 to 298) expressed in Escherichia coli using fluorescein diphosphate as substrate by fluores... |
Bioorg Med Chem Lett 29: 2358-2363 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.011
BindingDB Entry DOI: 10.7270/Q2280C4B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50612104

(CHEMBL5290902) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of flag-tagged PTP1B (1 to 321 residues) (unknown origin) expressed in bacterial expression system by UV/Vis spectrophotometry |
Bioorg Med Chem 24: 3343-52 (2016)
Article DOI: 10.1016/j.bmc.2016.06.035
BindingDB Entry DOI: 10.7270/Q20Z756P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142323
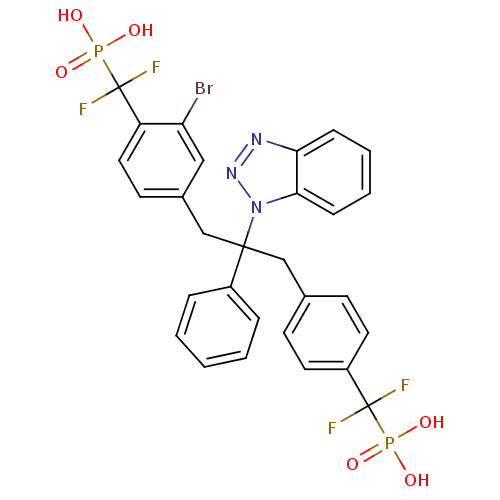
(CHEMBL267488 | [(4-{2-Benzotriazol-1-yl-3-[3-bromo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(c(Br)c2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C29H24BrF4N3O6P2/c30-24-16-20(12-15-23(24)29(33,34)45(41,42)43)18-27(21-6-2-1-3-7-21,37-26-9-5-4-8-25(26)35-36-37)17-19-10-13-22(14-11-19)28(31,32)44(38,39)40/h1-16H,17-18H2,(H2,38,39,40)(H2,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362181
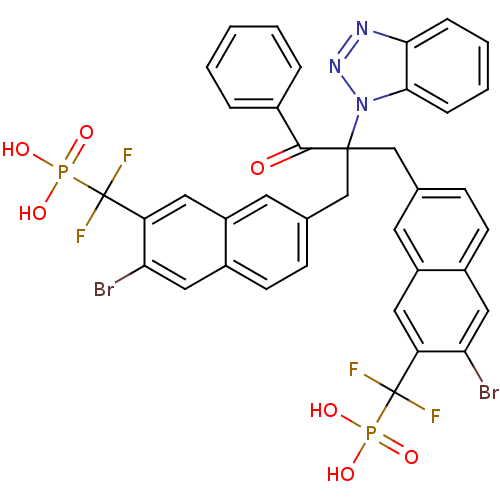
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362181

(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
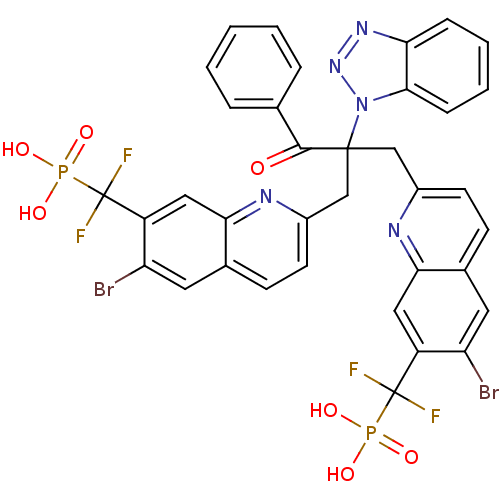
(Homo sapiens (Human)) | BDBM124220
(({2-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C36H25Br2F4N5O7P2/c37-27-14-21-10-12-23(43-30(21)16-25(27)35(39,40)55(49,50)51)18-34(33(48)20-6-2-1-3-7-20,47-32-9-5-4-8-29(32)45-46-47)19-24-13-11-22-15-28(38)26(17-31(22)44-24)36(41,42)56(52,53)54/h1-17H,18-19H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13604

(6-{4-[2-(1H-1,2,3-benzotriazol-1-yl)-3-{4-[difluor...)Show SMILES COC(CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein tyrosine phosphatase 1B (PTP1B) was determined in fluorescein diphosphate (FDP) assay |
Bioorg Med Chem Lett 14: 1043-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.076
BindingDB Entry DOI: 10.7270/Q24M9404 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50308854

(CHEMBL590235 | [7-(4-{1-Benzotriazol-1-yl-2-[4-(di...)Show SMILES COC(CC(C)C)c1ccc2ccc(-c3ccc(cc3)C(Cc3ccc(cc3)C(F)(F)P(O)(O)=O)(c3ccccc3)n3nnc4ccccc34)c(c2n1)P(O)(O)=O Show InChI InChI=1S/C42H40F2N4O7P2/c1-27(2)25-38(55-3)36-24-18-30-17-23-34(40(39(30)45-36)56(49,50)51)29-15-21-32(22-16-29)41(31-9-5-4-6-10-31,48-37-12-8-7-11-35(37)46-47-48)26-28-13-19-33(20-14-28)42(43,44)57(52,53)54/h4-24,27,38H,25-26H2,1-3H3,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B |
J Med Chem 53: 2333-44 (2010)
Article DOI: 10.1021/jm901090b
BindingDB Entry DOI: 10.7270/Q28P60MB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
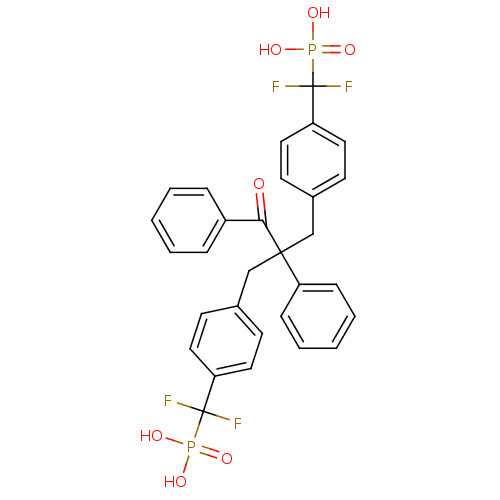
(Homo sapiens (Human)) | BDBM50142311
(CHEMBL266056 | [(4-{2-[4-(Difluoro-phosphono-methy...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C30H26F4O7P2/c31-29(32,42(36,37)38)25-15-11-21(12-16-25)19-28(24-9-5-2-6-10-24,27(35)23-7-3-1-4-8-23)20-22-13-17-26(18-14-22)30(33,34)43(39,40)41/h1-18H,19-20H2,(H2,36,37,38)(H2,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50612064

(CHEMBL5285288) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
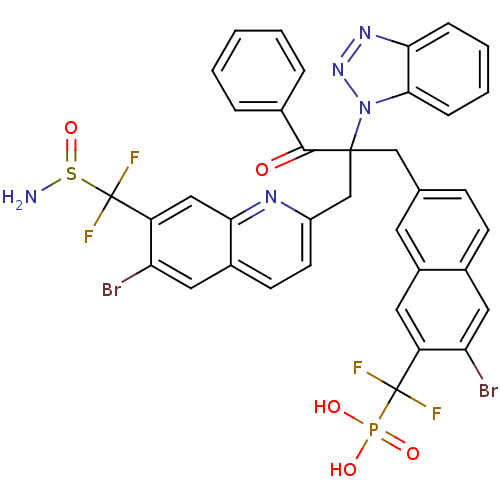
(Homo sapiens (Human)) | BDBM124217
(({7-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C37H26Br2F4N5O5PS/c38-29-16-23-11-10-21(14-25(23)15-27(29)36(40,41)54(50,51)52)19-35(34(49)22-6-2-1-3-7-22,48-33-9-5-4-8-31(33)46-47-48)20-26-13-12-24-17-30(39)28(18-32(24)45-26)37(42,43)55(44)53/h1-18H,19-20,44H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
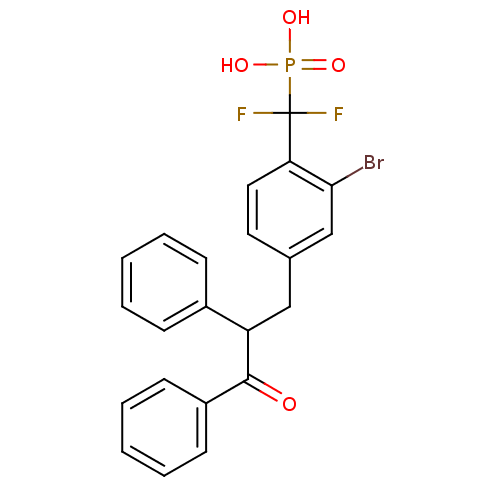
(Homo sapiens (Human)) | BDBM50142317
((2-bromo-4-(3-oxo-2,3-diphenylpropyl)phenyl)difluo...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Br Show InChI InChI=1S/C22H18BrF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50243184
({[2-Bromo-4-(40-bromo-30-sulfamoylbiphenyl-4-ylmet...)Show SMILES NS(=O)(=O)c1cc(ccc1Br)-c1ccc(CSCc2ccc(c(Br)c2)C(F)(F)P([O-])([O-])=O)cc1 Show InChI InChI=1S/C21H18Br2F2NO5PS2/c22-18-8-6-16(10-20(18)34(26,30)31)15-4-1-13(2-5-15)11-33-12-14-3-7-17(19(23)9-14)21(24,25)32(27,28)29/h1-10H,11-12H2,(H2,26,30,31)(H2,27,28,29)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B expressed in Escherichia coli BL21(DE3) cells |
Bioorg Med Chem 16: 6764-77 (2008)
Article DOI: 10.1016/j.bmc.2008.05.062
BindingDB Entry DOI: 10.7270/Q2930V2P |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50142318

(CHEMBL428651 | {[2-Chloro-4-(3-oxo-2,3-diphenyl-pr...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1Cl Show InChI InChI=1S/C22H18ClF2O4P/c23-20-14-15(11-12-19(20)22(24,25)30(27,28)29)13-18(16-7-3-1-4-8-16)21(26)17-9-5-2-6-10-17/h1-12,14,18H,13H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50500147
(CHEMBL3746639)Show SMILES CO[C@@H](CC(C)C)c1ccc2cc(cc(c2n1)P(O)(O)=O)-c1ccc(C[C@](Cc2ccc(cc2)C(F)(F)P(O)(O)=O)(c2ccccc2)n2nnc3ccccc23)cc1 |r| Show InChI InChI=1S/C43H42F2N4O7P2/c1-28(2)23-39(56-3)37-22-19-32-24-33(25-40(41(32)46-37)57(50,51)52)31-17-13-29(14-18-31)26-42(34-9-5-4-6-10-34,49-38-12-8-7-11-36(38)47-48-49)27-30-15-20-35(21-16-30)43(44,45)58(53,54)55/h4-22,24-25,28,39H,23,26-27H2,1-3H3,(H2,50,51,52)(H2,53,54,55)/t39-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) |
J Med Chem 58: 9063-88 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00586
BindingDB Entry DOI: 10.7270/Q24T6NC6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data