

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101858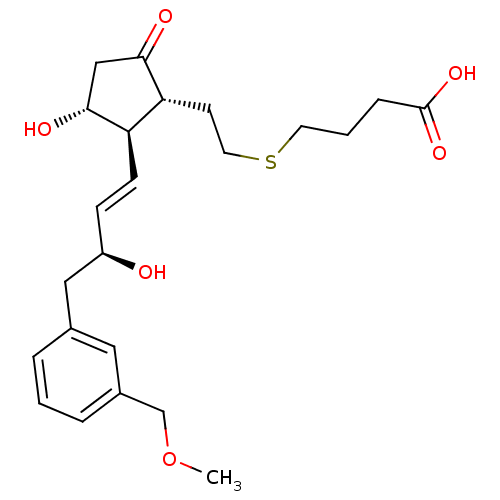 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50318871 (4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Agonist activity at mouse EP4 receptor expressed in CHO cells assessed as cAMP production | J Med Chem 53: 4332-53 (2010) Article DOI: 10.1021/jm9018756 BindingDB Entry DOI: 10.7270/Q21R6QPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101853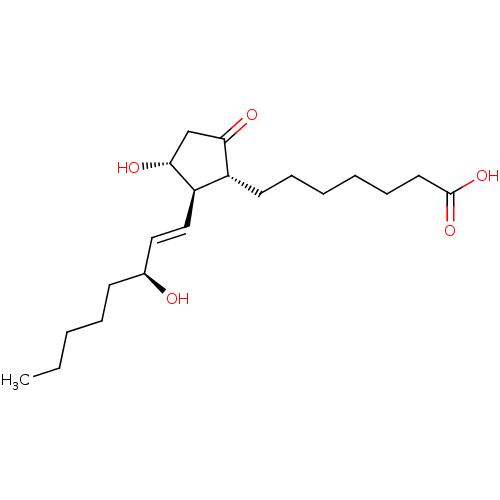 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101852 ((3-{(1R,2S,3R)-2-[(E)-(S)-4-(3-Ethoxymethyl-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101853 ((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101847 (CHEMBL305568 | {3-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101851 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Evaluated for its competitive binding affinity towards mouse Prostanoid EP4 receptor in CHO cells expressing prostanoid receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101856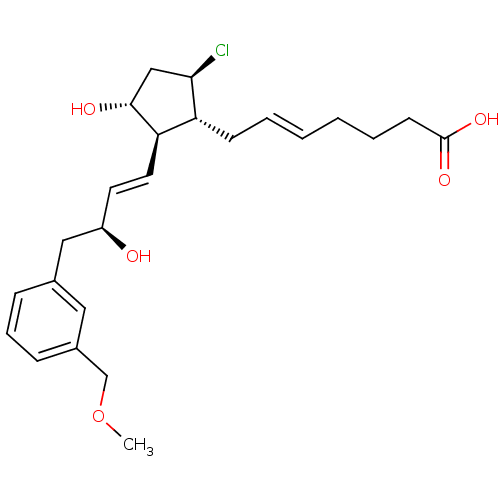 ((E)-7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101861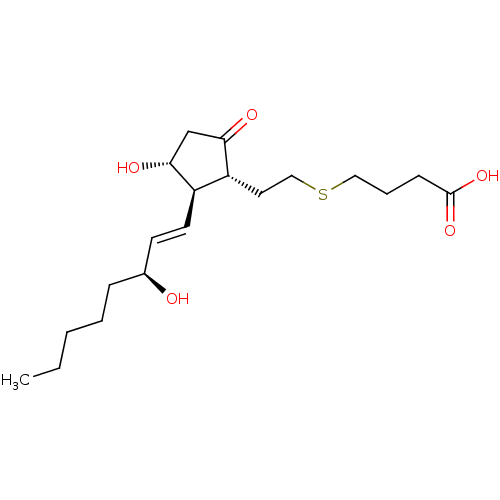 (4-{(S)-2-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101836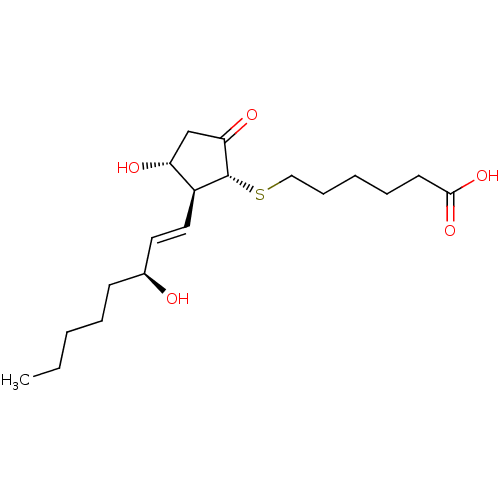 (6-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101836 (6-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Evaluated for its competitive binding affinity towards mouse Prostanoid EP4 receptor in CHO cells expressing prostanoid receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101842 (CHEMBL64217 | {3-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Evaluated for its competitive binding affinity towards mouse Prostanoid EP4 receptor in CHO cells expressing prostanoid receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101846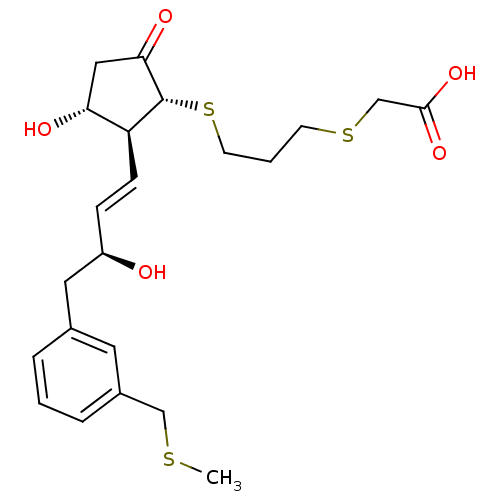 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101850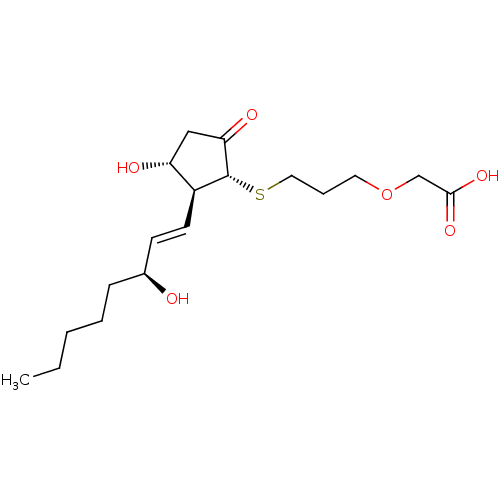 (CHEMBL64254 | {3-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101839 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101845 (CHEMBL62779 | [3-((1R,2S,3R)-3-Hydroxy-2-{(E)-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101857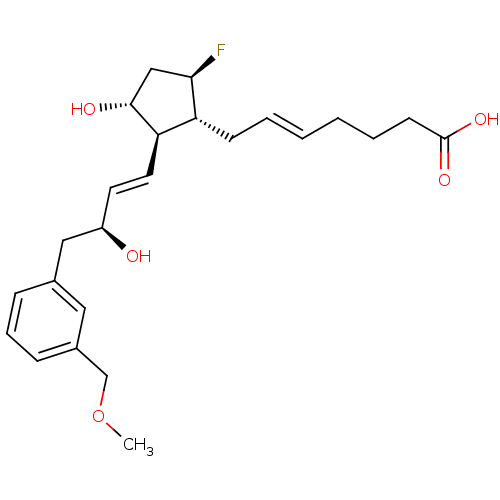 ((E)-7-{(1R,2R,3R,5R)-5-Fluoro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101864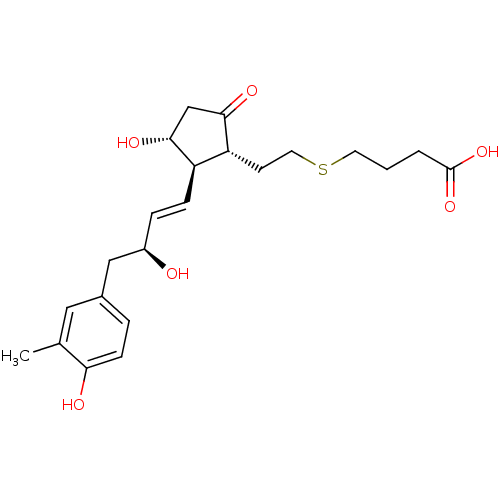 (4-(2-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101838 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101843 (CHEMBL64542 | {3-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101844 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101835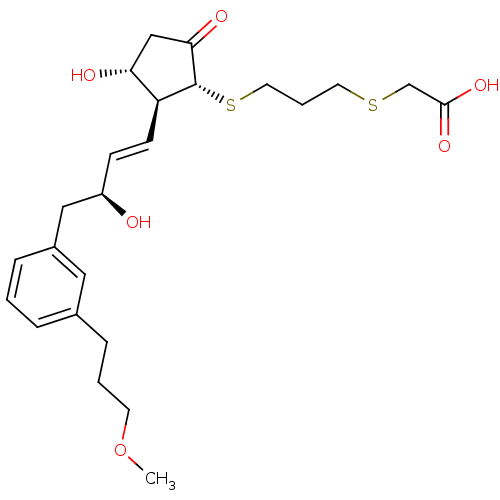 (CHEMBL303532 | [3-((1R,2S,3R)-3-Hydroxy-2-{(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101865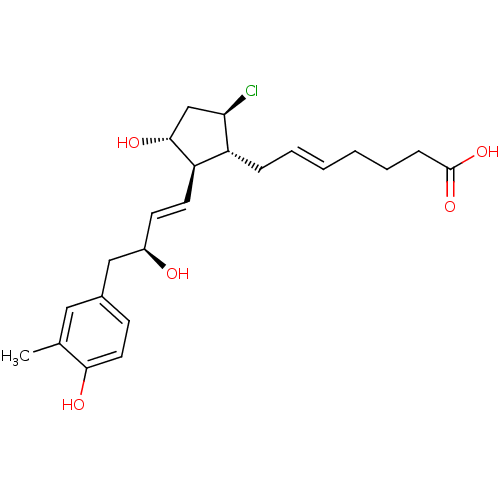 ((E)-7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101860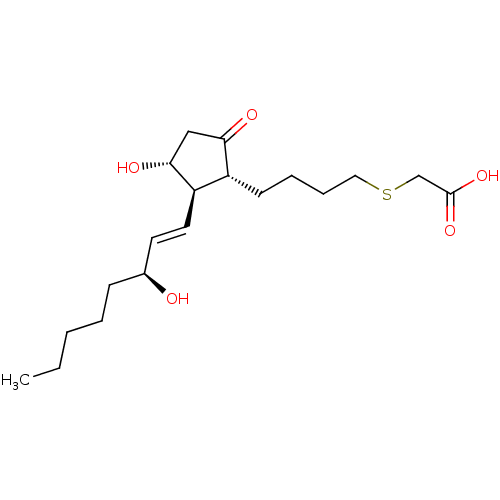 (CHEMBL64188 | {(S)-4-[(R)-3-Hydroxy-2-((1R,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 46 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101855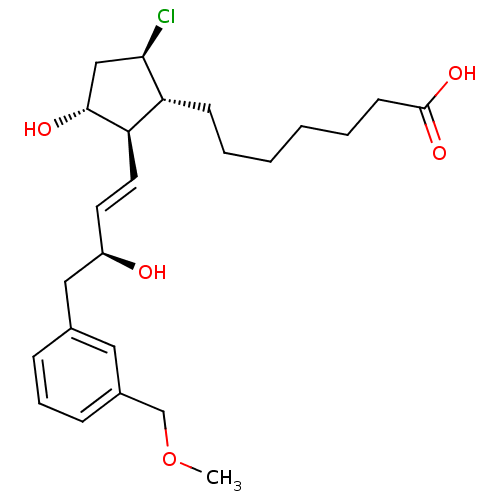 (7-{(1R,2R,3R,5R)-5-Chloro-3-hydroxy-2-[(E)-(S)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101849 (3-{2-[(S)-(R)-3-Hydroxy-2-((1S,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101859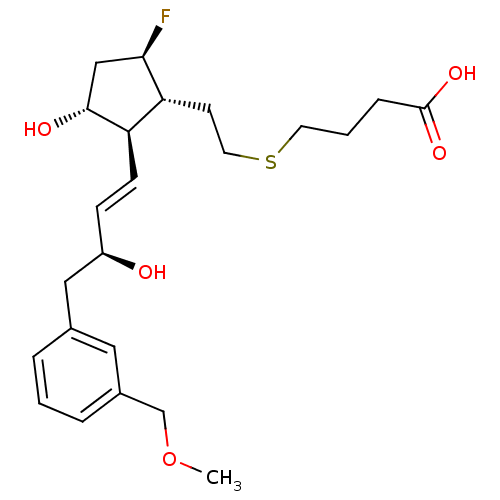 (4-(2-{(1R,2R,3R,5R)-5-Fluoro-3-hydroxy-2-[(E)-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for human Prostanoid IP receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101854 (CHEMBL59921 | {3-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 270 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101837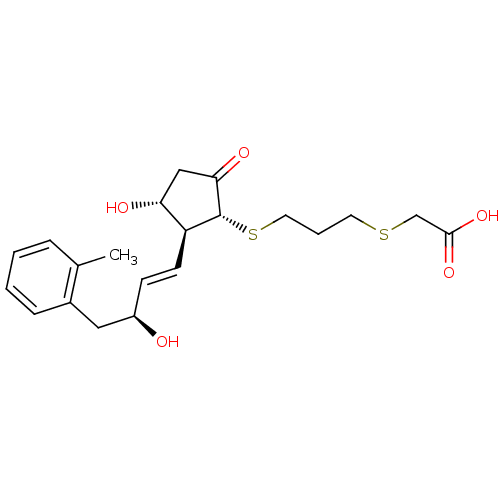 (CHEMBL303787 | {3-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101841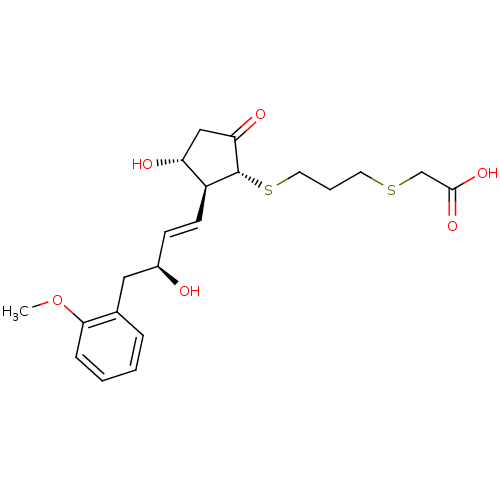 ((3-{(1R,2S,3R)-3-Hydroxy-2-[(E)-(S)-3-hydroxy-4-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101863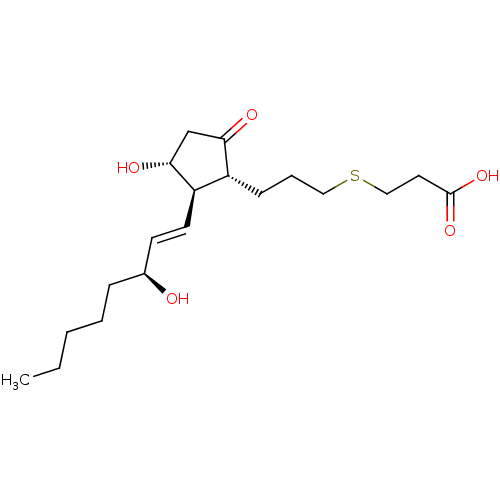 (3-{(S)-3-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101862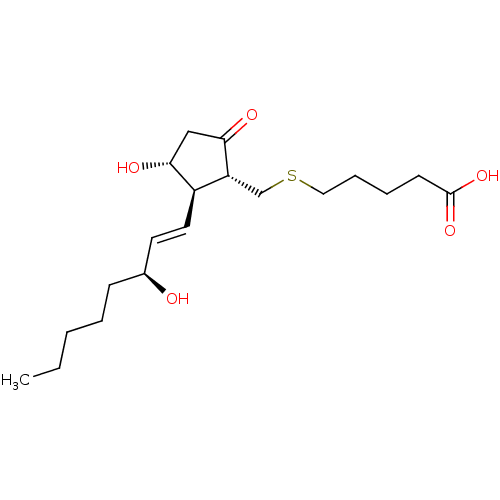 (5-[(S)-(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration for increased intracellular c-AMP production by mouse Prostanoid EP4 receptor | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101840 (CHEMBL64598 | {2-[(S)-(R)-3-Hydroxy-2-((1R,2R)-3-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Evaluated for its competitive binding affinity towards mouse Prostanoid EP4 receptor in CHO cells expressing prostanoid receptor | Bioorg Med Chem Lett 11: 2029-31 (2001) BindingDB Entry DOI: 10.7270/Q2833R9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||